Which Element Has The Highest Ionization Energy
Muz Play
Mar 21, 2025 · 5 min read
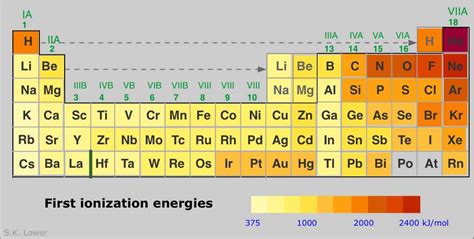
Table of Contents
Which Element Has the Highest Ionization Energy? Unraveling the Mysteries of Atomic Structure
The quest to identify the element boasting the highest ionization energy delves into the fascinating world of atomic structure and the forces governing electron behavior. Ionization energy, a fundamental concept in chemistry and physics, represents the minimum energy required to remove the most loosely bound electron from a neutral gaseous atom. This seemingly simple definition opens a door to a complex interplay of factors influencing an atom's tenacity in holding onto its electrons. Understanding these factors is crucial to comprehending why certain elements exhibit exceptionally high ionization energies.
The Factors Governing Ionization Energy
Several key factors determine an atom's ionization energy:
1. Nuclear Charge: The Stronger Pull
The nuclear charge, or the number of protons in the nucleus, plays a dominant role. A higher nuclear charge exerts a stronger electrostatic attraction on the electrons, making it harder to remove them. This directly translates to a higher ionization energy. The more protons tugging on the electrons, the tighter the electrons are held. Think of it like a magnet – the stronger the magnet (higher nuclear charge), the harder it is to pull something away (remove an electron).
2. Atomic Radius: Distance Matters
Atomic radius significantly influences ionization energy. A smaller atomic radius implies that the outermost electrons are closer to the positively charged nucleus. This proximity leads to a stronger electrostatic attraction, making it more challenging to remove an electron. Consequently, smaller atoms generally exhibit higher ionization energies. The closer the electron is to the nucleus, the stronger the grip.
3. Shielding Effect: Inner Electrons' Influence
The shielding effect arises from inner electrons repelling the outermost electrons. These inner electrons partially shield the outermost electrons from the full positive charge of the nucleus. The greater the number of inner electrons, the more effectively they shield the outermost electrons, reducing the effective nuclear charge experienced by these outer electrons. This shielding effect diminishes the attractive force between the nucleus and the outermost electrons, resulting in a lower ionization energy.
4. Electron Configuration: Stability and Subshells
The electron configuration profoundly impacts ionization energy. Atoms with completely filled or half-filled subshells (like noble gases and elements with half-filled p or d orbitals) possess enhanced stability. Removing an electron from such a stable configuration requires significantly more energy compared to removing an electron from a less stable configuration. This extra stability contributes to higher ionization energies.
Helium: The Champion of High Ionization Energy
Considering these factors, we can understand why helium (He) holds the title for the highest first ionization energy among all elements. Helium's unique electronic configuration contributes significantly to its exceptionally high ionization energy:
-
High Nuclear Charge: Helium possesses a nuclear charge of +2, a relatively high charge for a light element. This strong positive charge exerts a powerful attraction on its two electrons.
-
Small Atomic Radius: Helium has a remarkably small atomic radius. The two electrons are extremely close to the nucleus, experiencing a very strong electrostatic pull.
-
Minimal Shielding: Helium has no inner electrons to shield its outer electrons. The full nuclear charge is experienced by the outer electrons, maximizing the attraction.
-
Stable Electron Configuration: Helium's electron configuration (1s²) represents a completely filled electron shell, making it exceptionally stable. Removing an electron disrupts this stability, requiring a large amount of energy.
Trends in Ionization Energy Across the Periodic Table
Understanding the factors affecting ionization energy helps us predict trends across the periodic table:
-
Across a Period (Left to Right): Ionization energy generally increases as you move from left to right across a period. This is because the nuclear charge increases while the shielding effect remains relatively constant, leading to a stronger attraction between the nucleus and the outermost electrons.
-
Down a Group (Top to Bottom): Ionization energy generally decreases as you move down a group. This is primarily due to the increase in atomic radius. The increased distance between the nucleus and the outermost electrons weakens the electrostatic attraction, making it easier to remove an electron.
Beyond the First Ionization Energy: Successive Ionizations
It's important to note that ionization energy is not a single value. Each atom can undergo successive ionizations, where each subsequent electron removal requires progressively more energy. The second ionization energy is always higher than the first, the third higher than the second, and so on. This is because removing an electron alters the electron-to-proton ratio, making it more difficult to remove subsequent electrons due to the increased positive charge of the ion.
For example, removing a second electron from helium would require significantly more energy than removing the first electron. This is because removing the first electron creates a He⁺ ion, where the remaining electron experiences the full +2 nuclear charge without any shielding.
Applications and Significance of Ionization Energy
Ionization energy is not just a theoretical concept; it has widespread practical applications:
-
Spectroscopy: The energy required to ionize an atom is directly related to the wavelengths of light it absorbs or emits. This forms the basis of atomic spectroscopy, a powerful technique used to identify elements and analyze their composition.
-
Chemical Bonding: Ionization energy plays a crucial role in determining the nature of chemical bonds. Elements with low ionization energies readily lose electrons to form cations, while elements with high ionization energies tend to gain electrons to form anions.
-
Materials Science: Understanding ionization energies helps in designing materials with specific properties. For example, the ionization energy of elements dictates their behavior in semiconductor devices and their suitability for various applications.
-
Plasma Physics: Ionization energy is vital in plasma physics, where extremely high temperatures cause atoms to lose electrons and form plasmas. This understanding is critical in fields like fusion research and plasma-based technologies.
Conclusion: Helium's Reign Supreme
In conclusion, helium stands as the undisputed champion of elements with the highest first ionization energy. This remarkable property stems from its unique combination of high nuclear charge, small atomic radius, minimal shielding, and a highly stable electron configuration. Understanding the factors influencing ionization energy provides invaluable insights into the fundamental properties of atoms and their behavior in various chemical and physical processes. The principles discussed here are fundamental to chemistry, physics, and materials science, highlighting the importance of this seemingly simple concept. Further exploration of ionization energies and their relationship to atomic structure continues to unveil the intricacies of the quantum world and drive advancements in numerous scientific and technological fields.
Latest Posts
Latest Posts
-
1 2 Vs 1 4 Addition
Mar 21, 2025
-
Definition Of Cultural Lag In Sociology
Mar 21, 2025
-
Kinetic Energy Of A Spring Formula
Mar 21, 2025
-
Programmed And Non Programmed Decision Making
Mar 21, 2025
-
Chart Of Endocrine Glands And Their Hormones
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Which Element Has The Highest Ionization Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
