Which Experiment Deduced Charge On Electron
Muz Play
Mar 31, 2025 · 6 min read
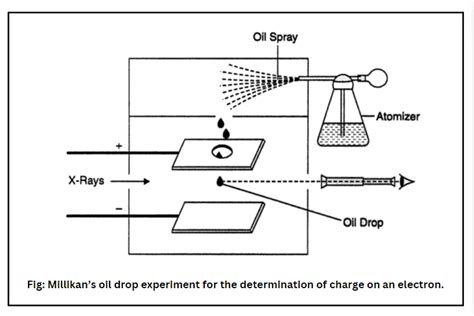
Table of Contents
Which Experiment Deduced the Charge on an Electron?
The determination of the charge of an electron, a fundamental constant in physics, was a monumental achievement in scientific history. While the existence of the electron was postulated earlier, pinning down its precise charge required ingenious experimentation and meticulous analysis. This wasn't a single experiment but a culmination of work, with Robert Millikan's oil drop experiment standing out as the pivotal study that definitively determined the elementary charge. This article delves deep into the history, methodology, and significance of Millikan's experiment, and also briefly touches upon the preceding research that paved the way for this breakthrough.
The Path to Millikan: Early Investigations and the Concept of Quantized Charge
Before Millikan, scientists were already grappling with the concept of a fundamental unit of electric charge. J.J. Thomson's cathode ray tube experiments in the late 1890s provided compelling evidence for the existence of the electron, a negatively charged particle much smaller than an atom. However, Thomson's work didn't precisely measure the electron's charge. He could determine the charge-to-mass ratio (e/m) of the electron, but not the charge (e) or mass (m) independently. This meant that while the existence of the electron was established, its fundamental properties remained elusive.
Several other physicists attempted to measure the elementary charge, utilizing various methods such as charged water droplets and clouds. These early experiments, although not yielding precise results, provided crucial insights and laid the groundwork for Millikan's groundbreaking work. They highlighted the inherent challenges in manipulating and measuring the charge of such incredibly tiny particles. The difficulty stemmed from the erratic behavior of charged particles influenced by Brownian motion (random movement due to collisions with air molecules) and other environmental factors. The need for a more controlled and refined experimental setup was clear.
Millikan's Oil Drop Experiment: A Masterpiece of Experimental Design
Robert Millikan's oil drop experiment, conducted between 1909 and 1913, elegantly addressed the shortcomings of previous attempts. His ingenious approach involved meticulously controlling the movement of charged oil droplets in an electric field, allowing for precise measurements of their charge. The experiment's brilliance lies in its simplicity and elegance, combined with meticulous control over experimental variables.
The Experimental Setup: A Symphony of Precision
The apparatus consisted of a pair of horizontal parallel plates, creating a uniform electric field between them. Oil droplets were sprayed into the chamber above the plates. Some of these droplets became charged through friction during the atomization process. A microscope was used to observe the motion of individual oil droplets falling under the influence of gravity and the electric field.
By adjusting the electric field, Millikan could precisely balance the gravitational force on a droplet with the upward electrical force, causing the droplet to remain suspended in mid-air. This allowed him to measure the droplet's charge directly. He observed that the droplets did not fall at completely constant speeds, due to slight changes in the charge, but this allowed him to develop a better way of measuring the charge.
Analyzing the Data: Unraveling the Quantized Nature of Charge
Millikan meticulously measured the terminal velocity (constant speed) of the droplets both when they were falling under gravity alone and when they were suspended or moving under the influence of the electric field. By applying Stokes' law (which describes the frictional force on a sphere moving through a fluid), he could calculate the droplet's radius and mass. Knowing the mass and the electric field strength, he could determine the charge on each droplet.
The astonishing result was that the charges on all the droplets were integer multiples of a single fundamental charge. This demonstrated that electric charge is quantized – it exists in discrete units, not as a continuous variable. This fundamental unit was identified as the elementary charge, the charge of a single electron, approximately 1.602 x 10⁻¹⁹ coulombs. The fact that all measured charges were multiples of this value provided irrefutable evidence for the quantization of electric charge.
Significance of Millikan's Experiment: A Cornerstone of Modern Physics
Millikan's oil drop experiment had a profound impact on physics, establishing several key principles:
-
Quantization of Charge: The most significant outcome was the definitive proof of the quantization of electric charge. This fundamental concept is crucial to our understanding of the atomic structure and the behavior of matter at the microscopic level. It forms the basis of our understanding of electric current and countless other electrical phenomena.
-
Precise Measurement of the Electron's Charge: The experiment yielded an accurate measurement of the elementary charge (e), a fundamental physical constant. This value is essential for many calculations in atomic and nuclear physics, as well as other related fields.
-
Confirmation of the Electron's Existence: While Thomson's work had already suggested the existence of the electron, Millikan's experiment provided further compelling evidence, solidifying the electron's place as a fundamental constituent of matter.
-
Advancement of Experimental Techniques: Millikan's meticulous experimental design and sophisticated analysis techniques significantly advanced experimental physics, inspiring future generations of physicists to pursue increasingly precise measurements. His approach became a model for conducting rigorous scientific experiments.
-
Foundation for Further Research: The precise measurement of the electron's charge opened up new avenues of research. It paved the way for better understanding of atomic structure, the development of quantum mechanics, and numerous other advancements in physics and related fields.
Challenges and Criticisms of Millikan's Work
While Millikan's experiment is celebrated as a landmark achievement, it's not without its share of historical debate. Some criticisms have been raised regarding his data analysis and the selection of data points used in his calculations. Some argue that Millikan selectively chose data that supported his hypothesis, potentially overlooking or discarding data that deviated from the expected results. However, this criticism doesn't negate the fundamental validity of his conclusions. Subsequent experiments using improved techniques and larger datasets have confirmed Millikan's findings. The criticism primarily serves as a reminder that even the most groundbreaking scientific work is subject to scrutiny and refinement over time. Modern re-analyses of Millikan's data, taking into account the corrections needed for air viscosity and other corrections, have largely validated his original conclusions.
Conclusion: A Legacy of Precision and Insight
Robert Millikan's oil drop experiment remains a cornerstone of modern physics. Its elegant design, meticulous execution, and profound impact on our understanding of the fundamental nature of matter have secured its place in scientific history. Although some criticisms regarding the data analysis exist, these do not diminish the revolutionary significance of his work. The experiment not only provided a precise measurement of the electron's charge but also demonstrated the fundamental quantization of electric charge, a concept that underpins much of modern physics and our understanding of the universe. The experiment remains a testament to the power of innovative experimental design, rigorous data analysis, and the pursuit of fundamental scientific truths. It serves as an enduring example of how meticulous scientific work can unlock profound insights into the workings of the natural world.
Latest Posts
Latest Posts
-
Why Is Water Necessary For Life
Apr 02, 2025
-
The Process Of Independent Assortment Refers To
Apr 02, 2025
-
Is Table Salt Homogeneous Or Heterogeneous
Apr 02, 2025
-
What Part Of Bacteria Cell Helps It Move
Apr 02, 2025
-
Is The Organic Layer On The Top Or Bottom
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Which Experiment Deduced Charge On Electron . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
