Which Molecule Below Has Hydrogen Bonding
Muz Play
May 09, 2025 · 6 min read
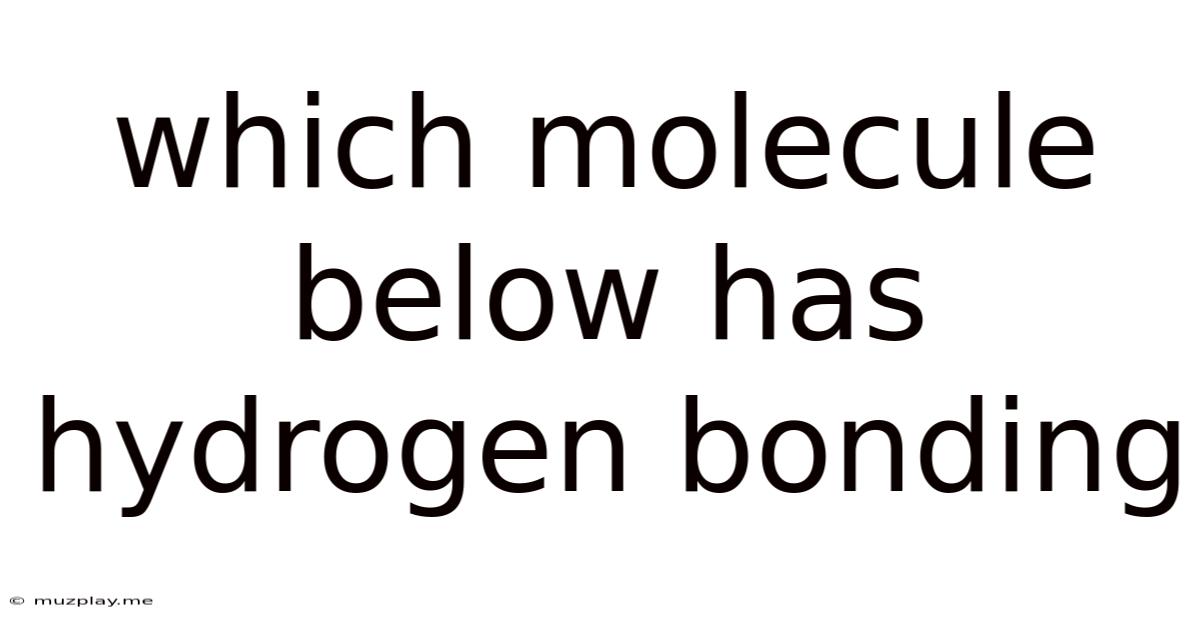
Table of Contents
Which Molecule Below Has Hydrogen Bonding? A Deep Dive into Hydrogen Bonds and Molecular Interactions
Hydrogen bonding, a special type of dipole-dipole attraction, significantly impacts the properties of many molecules. Understanding which molecules exhibit hydrogen bonding is crucial in various fields, from chemistry and biology to materials science and environmental studies. This article will explore the fundamentals of hydrogen bonding, providing a clear understanding of what constitutes a hydrogen bond and how to identify its presence in different molecules. We'll then delve into specific examples, clarifying which molecules possess this critical intermolecular force.
Understanding Hydrogen Bonds: The Basics
A hydrogen bond is a relatively strong type of intermolecular force that occurs between a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) and another electronegative atom in a different molecule (or a different part of the same molecule). This electronegativity difference creates a significant dipole moment. The hydrogen atom, being partially positive (δ+), is strongly attracted to the partially negative (δ-) lone pair of electrons on the electronegative atom of a neighboring molecule.
Key Requirements for Hydrogen Bonding:
-
A highly electronegative atom: This atom must be strongly electronegative, pulling electron density away from the hydrogen atom, creating a significant partial positive charge on the hydrogen. Oxygen (O), nitrogen (N), and fluorine (F) are the most common electronegative atoms involved in hydrogen bonding.
-
A hydrogen atom: This hydrogen atom must be covalently bonded to the highly electronegative atom mentioned above.
-
A lone pair of electrons: The electronegative atom in the second molecule (or part of the molecule) must possess a lone pair of electrons to accept the hydrogen bond. This electron pair is attracted to the partially positive hydrogen atom.
Distinguishing Hydrogen Bonds from Covalent Bonds:
It's crucial to distinguish between hydrogen bonds and covalent bonds. While both involve interactions between atoms, they differ significantly in strength and nature:
-
Covalent bonds: These are strong intramolecular forces involving the sharing of electron pairs between atoms. They hold atoms together within a molecule.
-
Hydrogen bonds: These are weaker intermolecular forces involving the attraction between a partially positive hydrogen atom and a partially negative atom. They exist between different molecules or different parts of the same molecule.
The strength of a hydrogen bond is generally weaker than a covalent bond but significantly stronger than other intermolecular forces like van der Waals forces or dipole-dipole interactions. This strength has profound consequences for the physical and chemical properties of substances.
Identifying Molecules with Hydrogen Bonds: Practical Examples
Let's consider several examples to illustrate the presence or absence of hydrogen bonding. We'll focus on the key requirements outlined above.
Molecules exhibiting hydrogen bonding:
-
Water (H₂O): Water is the quintessential example of hydrogen bonding. The oxygen atom is highly electronegative, creating a partially positive charge on each hydrogen atom. Each oxygen atom also has two lone pairs of electrons, capable of accepting hydrogen bonds from hydrogen atoms in other water molecules. This extensive hydrogen bonding network is responsible for water's high boiling point, surface tension, and its ability to act as a universal solvent.
-
Ammonia (NH₃): Nitrogen is also highly electronegative. The hydrogen atoms in ammonia carry a partial positive charge, and the nitrogen atom has a lone pair of electrons, readily forming hydrogen bonds with hydrogen atoms in other ammonia molecules.
-
Hydrogen fluoride (HF): Fluorine is the most electronegative element. The hydrogen atom in HF carries a strong partial positive charge, and the fluorine atom has three lone pairs of electrons, leading to strong hydrogen bonding.
-
Ethanol (CH₃CH₂OH): The hydroxyl group (-OH) in ethanol is the key to its hydrogen bonding capability. The oxygen atom is electronegative, creating a partially positive hydrogen atom, while the oxygen also possesses lone pairs of electrons for hydrogen bond acceptance.
-
Carboxylic acids (RCOOH): Carboxylic acids contain both a hydroxyl group (-OH) and a carbonyl group (C=O). The hydroxyl group participates in hydrogen bonding, contributing to the high boiling points and other unique properties of these compounds.
Molecules lacking significant hydrogen bonding:
-
Methane (CH₄): Carbon is not sufficiently electronegative to create a significant partial positive charge on the hydrogen atoms. Therefore, methane molecules primarily interact through weak van der Waals forces.
-
Carbon dioxide (CO₂): Although oxygen is electronegative, the carbon atom is located symmetrically between the two oxygen atoms, resulting in a non-polar molecule with minimal dipole moment. Thus, hydrogen bonding is absent.
-
Chloromethane (CH₃Cl): Chlorine is more electronegative than carbon, but the electronegativity difference is not large enough to create the strong partial positive charge on hydrogen needed for hydrogen bonding. The primary intermolecular forces are dipole-dipole interactions and van der Waals forces.
-
Benzene (C₆H₆): Benzene is a nonpolar molecule, lacking the electronegative atom-hydrogen combination required for hydrogen bonding.
The Impact of Hydrogen Bonding on Physical Properties
The presence or absence of hydrogen bonding profoundly influences various physical properties of molecules:
-
Boiling point: Molecules capable of hydrogen bonding generally have higher boiling points than molecules of comparable size and molecular weight that lack hydrogen bonding. This is because the relatively strong hydrogen bonds require more energy to overcome during the boiling process.
-
Melting point: Similar to boiling point, hydrogen bonding contributes to higher melting points. The hydrogen bonds need to be broken to transition from the solid to the liquid phase.
-
Solubility: Hydrogen bonding plays a critical role in solubility. Molecules capable of hydrogen bonding tend to be more soluble in polar solvents like water, as they can form hydrogen bonds with water molecules.
-
Viscosity: The presence of hydrogen bonds can increase the viscosity of a liquid, as the intermolecular forces hinder the molecules' movement.
-
Surface tension: Hydrogen bonding contributes to high surface tension, as it creates strong cohesive forces within the liquid.
Beyond the Basics: Factors Influencing Hydrogen Bond Strength
While the presence of a highly electronegative atom bonded to hydrogen is a prerequisite, other factors can influence the strength of hydrogen bonds:
-
Electronegativity: The higher the electronegativity difference between the hydrogen and the electronegative atom, the stronger the hydrogen bond. Fluorine, being the most electronegative element, forms the strongest hydrogen bonds.
-
Geometry: The spatial arrangement of atoms influences hydrogen bond strength. Linear arrangements generally lead to stronger bonds than bent arrangements.
-
Steric hindrance: Bulky groups surrounding the electronegative atom can hinder the formation of hydrogen bonds by creating steric hindrance.
-
Solvent effects: The solvent in which the molecules are dissolved can affect the strength of hydrogen bonds. Polar solvents can enhance hydrogen bonding, while nonpolar solvents can weaken it.
Conclusion: A Vital Intermolecular Force
Hydrogen bonding is a fundamental intermolecular force with significant consequences for the physical and chemical properties of molecules. Understanding the requirements for hydrogen bonding – a highly electronegative atom (O, N, or F) covalently bonded to hydrogen, and the presence of a lone pair of electrons on another electronegative atom – is key to predicting and explaining the behavior of various substances. The strength of hydrogen bonds is not solely determined by the presence of these requirements but is also influenced by factors such as electronegativity, geometry, steric hindrance, and solvent effects. This comprehensive understanding of hydrogen bonding is essential across numerous scientific disciplines. The examples provided in this article illustrate the diverse range of molecules affected by this crucial intermolecular interaction and highlight its profound impact on the macroscopic properties we observe.
Latest Posts
Latest Posts
-
What Membrane Holds The Coils Of The Small Intestine Together
May 10, 2025
-
What Makes Something A Good Nucleophile
May 10, 2025
-
Finding The Resistance Of A Wire
May 10, 2025
-
What Happens To Water Molecules In Light Reactions
May 10, 2025
Related Post
Thank you for visiting our website which covers about Which Molecule Below Has Hydrogen Bonding . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.