Which Of The Following Ions Would Be Considered An Anion
Muz Play
Mar 17, 2025 · 6 min read
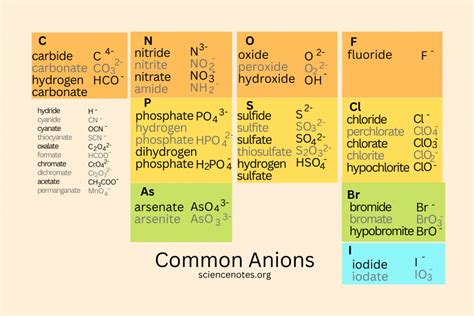
Table of Contents
Which of the Following Ions Would Be Considered an Anion? A Comprehensive Guide
Understanding the difference between anions and cations is fundamental to grasping the basics of chemistry. This article will delve deep into the concept of anions, exploring their characteristics, formation, and providing numerous examples to solidify your understanding. We'll also look at how to identify anions amongst a group of ions, answering the question: which of the following ions would be considered an anion?
Understanding Anions: The Basics
Anions are negatively charged ions. This negative charge arises from the gain of one or more electrons. Remember, atoms are inherently electrically neutral; they possess an equal number of protons (positively charged particles in the nucleus) and electrons (negatively charged particles orbiting the nucleus). When an atom gains electrons, it acquires a negative charge, transforming it into an anion. The magnitude of the negative charge depends on the number of electrons gained. For example, gaining one electron results in a -1 charge (e.g., Cl⁻), while gaining two electrons results in a -2 charge (e.g., O²⁻).
The Formation of Anions: A Closer Look
Anion formation is often driven by the atom's desire to achieve a stable electron configuration, typically resembling that of a noble gas. Noble gases are chemically inert due to their full valence electron shells. Atoms, particularly those in Groups 16 and 17 (chalcogens and halogens, respectively), readily gain electrons to achieve this stable octet configuration.
Let's examine a classic example: chlorine (Cl). Chlorine has seven valence electrons. By gaining one electron, it completes its outermost shell, achieving a stable octet similar to argon (Ar). This process transforms the neutral chlorine atom into a chloride anion (Cl⁻).
Other factors influencing anion formation include:
- Electronegativity: Atoms with high electronegativity – a measure of an atom's ability to attract electrons – are more likely to gain electrons and form anions. Elements towards the right and top of the periodic table generally exhibit higher electronegativity.
- Ionization Energy: While related to cation formation (loss of electrons), ionization energy also indirectly influences anion formation. A lower ionization energy for the second electron added can sometimes make the process more favorable compared to the initial gain of the first electron.
Identifying Anions: A Practical Approach
When faced with a list of ions, identifying the anions involves a simple yet crucial step: look for the negative charge. Any ion carrying a negative charge (indicated by a superscript minus sign, e.g., -1, -2, -3) is an anion. Conversely, ions with a positive charge are cations.
Examples of Common Anions: Expanding Your Knowledge
Understanding anions requires familiarity with specific examples. Here are some common anions, categorized by their origin and charge:
Monatomic Anions (Single Atom Anions):
These anions consist of a single atom with a negative charge. They are typically formed by non-metal atoms.
- Halide ions: These are formed by the halogens (Group 17) and include fluoride (F⁻), chloride (Cl⁻), bromide (Br⁻), and iodide (I⁻). These are among the most common anions found in various chemical compounds.
- Oxide ion: Oxygen (O) readily forms the oxide ion (O²⁻) by gaining two electrons. Oxides are prevalent in many minerals and inorganic compounds.
- Sulfide ion: Sulfur (S) gains two electrons to form the sulfide ion (S²⁻). Sulfides are often encountered in minerals and some organic compounds.
- Nitride ion: Nitrogen (N) can gain three electrons to form the nitride ion (N³⁻). Nitrides are less common than oxides and sulfides but still have significance in certain materials.
- Phosphide ion: Phosphorus (P) can gain three electrons to form the phosphide ion (P³⁻).
Polyatomic Anions (Multiple Atom Anions):
These anions consist of two or more atoms covalently bonded together and carrying a net negative charge. They frequently involve non-metals.
- Hydroxide ion (OH⁻): A very common anion found in bases and many aqueous solutions.
- Nitrate ion (NO₃⁻): A crucial anion in fertilizers and many other chemical processes.
- Sulfate ion (SO₄²⁻): Another significant anion found in various minerals and chemical compounds.
- Phosphate ion (PO₄³⁻): A vital anion in biological systems and fertilizers.
- Carbonate ion (CO₃²⁻): A crucial component of limestone and other carbonate minerals.
- Bicarbonate ion (HCO₃⁻): Plays a vital role in maintaining blood pH.
- Acetate ion (CH₃COO⁻): An anion found in vinegar and many organic compounds.
- Permanganate ion (MnO₄⁻): A powerful oxidizing agent used in various chemical reactions and analytical techniques.
- Chromate ion (CrO₄²⁻) and Dichromate ion (Cr₂O₇²⁻): These anions have important roles in various industrial applications and chemical processes.
Distinguishing Anions from Cations: A Comparative Analysis
To fully grasp the concept of anions, it's essential to contrast them with cations. Here's a table summarizing the key differences:
| Feature | Anion | Cation |
|---|---|---|
| Charge | Negative (-) | Positive (+) |
| Electron Gain/Loss | Gains electrons | Loses electrons |
| Formation | Typically by non-metals | Typically by metals |
| Examples | Cl⁻, O²⁻, SO₄²⁻, NO₃⁻ | Na⁺, Ca²⁺, Fe³⁺, NH₄⁺ |
| Chemical Behavior | Often acts as a base or ligand | Often acts as an acid or complexing agent |
Answering the Question: Which of the Following Ions Would Be Considered an Anion?
Now, let's tackle the central question. Given a list of ions, you can easily identify the anions by looking for the negative charge. For example:
Example 1:
Which of the following ions are anions: Na⁺, Cl⁻, Ca²⁺, SO₄²⁻, Mg²⁺, NO₃⁻?
Answer: Cl⁻, SO₄²⁻, and NO₃⁻ are anions because they carry negative charges.
Example 2:
Identify the anions in this set: K⁺, O²⁻, Fe³⁺, PO₄³⁻, H⁺, OH⁻.
Answer: O²⁻, PO₄³⁻, and OH⁻ are anions due to their negative charges.
Example 3 (More Challenging):
Consider the following ions: NH₄⁺, CH₃COO⁻, MnO₄⁻, Al³⁺, CO₃²⁻. Which are anions?
Answer: CH₃COO⁻, MnO₄⁻, and CO₃²⁻ are the anions.
Remember, the presence of a negative charge is the definitive characteristic that identifies an ion as an anion.
Applications of Anions: Real-World Significance
Anions play crucial roles in numerous areas, spanning various scientific disciplines and industrial applications. Here are a few examples:
- Biological systems: Anions like phosphate (PO₄³⁻) and bicarbonate (HCO₃⁻) are essential components of biological molecules and metabolic processes. Chloride (Cl⁻) ions are critical in maintaining fluid balance and nerve function.
- Industrial processes: Many industrial processes utilize anions in various applications, including manufacturing fertilizers (nitrate), producing detergents (sulfate), and electroplating (chromate).
- Medicine: Anions are present in numerous medications and play vital roles in various physiological functions.
- Materials science: The properties of various materials, including ceramics and polymers, are heavily influenced by the presence of specific anions.
Conclusion: Mastering Anion Identification
Understanding anions is crucial for mastering fundamental chemistry principles. By focusing on the negative charge, recognizing common examples, and understanding their formation, you can confidently identify anions among various ion sets. The ability to distinguish anions from cations is vital for a comprehensive understanding of chemical reactions, biological processes, and numerous industrial applications. This detailed explanation provides you with a robust foundation for tackling more advanced chemistry concepts.
Latest Posts
Latest Posts
-
What Are The Physical Properties Of A Metal
Mar 18, 2025
-
Why Is The Force Subscript Not Written In The Us
Mar 18, 2025
-
A Relationship In Which Both Organisms Benefit
Mar 18, 2025
-
If One Of The Reactants In A Reaction Is
Mar 18, 2025
-
Is Melting Point Physical Or Chemical Property
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Ions Would Be Considered An Anion . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
