Which Of The Following Orbital Diagrams Represents A Diamagnetic Atom
Muz Play
Apr 04, 2025 · 5 min read
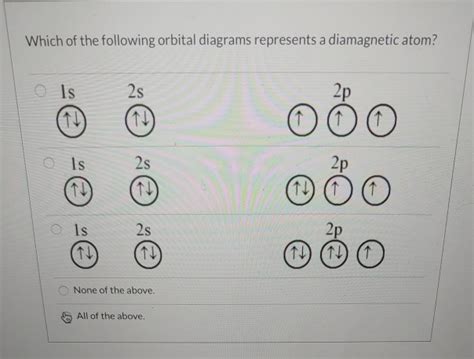
Table of Contents
Which of the Following Orbital Diagrams Represents a Diamagnetic Atom? A Deep Dive into Electron Configuration and Magnetism
Understanding the magnetic properties of atoms is crucial in various fields, from materials science to chemistry. This understanding hinges on the arrangement of electrons within an atom, specifically their spin and orbital occupation. This article will delve into the concept of diamagnetism and paramagnetism, explain how to interpret orbital diagrams, and ultimately determine which orbital diagrams represent diamagnetic atoms.
Diamagnetism vs. Paramagnetism: A Fundamental Difference
Before we tackle orbital diagrams, let's clarify the key difference between diamagnetism and paramagnetism. These properties describe how atoms behave in the presence of an external magnetic field:
-
Diamagnetism: This is a fundamental property of all matter. Diamagnetic materials are weakly repelled by an external magnetic field. This repulsion arises from the changes in the orbital motion of electrons induced by the external field. Crucially, diamagnetism is present in atoms with all paired electrons.
-
Paramagnetism: Paramagnetic materials are attracted to an external magnetic field. This attraction is significantly stronger than the diamagnetic repulsion. Paramagnetism occurs when an atom possesses unpaired electrons. These unpaired electrons possess a net magnetic moment which aligns with the external field, leading to the attraction.
Orbital Diagrams: Visualizing Electron Configuration
Orbital diagrams provide a visual representation of the electronic configuration of an atom. They show how electrons are distributed among different atomic orbitals, taking into account the Pauli Exclusion Principle (which states that no two electrons in an atom can have the same set of four quantum numbers) and Hund's Rule (which states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital).
Each orbital is represented by a box, and electrons are represented by arrows. An upward arrow signifies an electron with spin +1/2, while a downward arrow signifies an electron with spin -1/2. Paired electrons, with opposite spins, are crucial for diamagnetism.
Example: Consider the element Carbon (C). Its electron configuration is 1s²2s²2p². The orbital diagram would look like this:
1s: ↑↓
2s: ↑↓
2p: ↑ ↑ _
Note that the 2p subshell has two unpaired electrons. This means carbon is paramagnetic.
Identifying Diamagnetic Atoms from Orbital Diagrams
To identify a diamagnetic atom from its orbital diagram, look for the following:
-
All electrons are paired: Every orbital box must contain two arrows, one pointing up and one pointing down. There should be no unpaired electrons.
-
Complete subshells: Diamagnetic atoms often (but not always) have completely filled subshells (s², p⁶, d¹⁰, f¹⁴). However, it’s crucial to remember that this is a sufficient but not necessary condition for diamagnetism. An atom can be diamagnetic even if some subshells are not completely filled, as long as all electrons are paired.
Example of a Diamagnetic Atom:
Let's consider Neon (Ne), with an electron configuration of 1s²2s²2p⁶. Its orbital diagram:
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑↓
All electrons are paired, and all subshells are completely filled. Therefore, Neon is diamagnetic.
Analyzing Hypothetical Orbital Diagrams
Let's analyze several hypothetical orbital diagrams to determine which represents a diamagnetic atom. Remember, the key is the presence or absence of unpaired electrons.
Scenario 1:
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑ _
This atom has one unpaired electron in the 2p subshell. Therefore, it is paramagnetic, not diamagnetic.
Scenario 2:
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑↓
3s: ↑↓
All electrons are paired. Therefore, this atom is diamagnetic.
Scenario 3:
1s: ↑↓
2s: ↑↓
2p: ↑ ↑ ↑ _ _
This atom has three unpaired electrons in the 2p subshell. It is strongly paramagnetic.
Scenario 4:
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑
3s: ↑
This atom has two unpaired electrons (one in 2p and one in 3s). It is paramagnetic.
Scenario 5 (A more complex example):
1s: ↑↓
2s: ↑↓
2p: ↑↓ ↑↓ ↑↓
3s: ↑↓
3p: ↑↓ ↑↓ ↑↓
3d: ↑↓ ↑↓ ↑↓ ↑↓ ↑↓
4s: ↑↓
Even though not all subshells are fully filled, all electrons are paired. Therefore, this atom is diamagnetic.
Beyond Simple Atoms: Molecules and Ions
The principles discussed above apply equally well to molecules and ions. A molecule or ion is diamagnetic if all its electrons are paired. The determination requires constructing the molecular orbital diagram, a more advanced topic. However, the fundamental principle remains the same: the presence or absence of unpaired electrons dictates the magnetic properties.
Practical Applications of Diamagnetism and Paramagnetism
The magnetic properties of materials have significant applications in various fields:
-
Nuclear Magnetic Resonance (NMR) Spectroscopy: NMR uses the magnetic properties of atomic nuclei to determine the structure of molecules. Understanding diamagnetism and paramagnetism is essential for interpreting NMR spectra.
-
Magnetic Resonance Imaging (MRI): MRI relies on the magnetic properties of hydrogen nuclei in the body to create detailed images of internal organs and tissues.
-
Materials Science: The magnetic properties of materials are crucial in designing magnets, magnetic storage devices, and other magnetic technologies.
-
Chemical Analysis: Magnetic susceptibility measurements can be used to identify the presence of unpaired electrons in a compound, providing valuable information about its electronic structure.
Conclusion
Determining whether an atom is diamagnetic or paramagnetic is a fundamental concept in chemistry and physics. By carefully examining the orbital diagram and identifying the presence or absence of unpaired electrons, we can accurately classify atoms based on their magnetic behavior. Remember, diamagnetism is a property of all atoms due to electron orbital motion, but the presence of unpaired electrons makes paramagnetism the dominant magnetic effect. The ability to differentiate between these two magnetic properties is crucial for understanding the behavior of matter in magnetic fields and has numerous applications in various scientific and technological fields. This detailed analysis provides a strong foundation for further exploration into the fascinating world of atomic magnetism.
Latest Posts
Latest Posts
-
How Do You Measure Public Opinion
Apr 10, 2025
-
Magnetic Field Of A Dipole Formula
Apr 10, 2025
-
How To Describe The Distribution Of Data
Apr 10, 2025
-
What Is The Reaction Order With Respect To A
Apr 10, 2025
-
Find The Force Of Tension T In Each Rope
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Orbital Diagrams Represents A Diamagnetic Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.