Why Are Tertiary Carbocations More Stable
Muz Play
Mar 26, 2025 · 5 min read
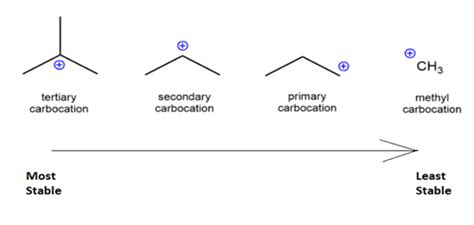
Table of Contents
Why Are Tertiary Carbocations More Stable? A Deep Dive into Carbocation Stability
Carbocations, organic intermediates carrying a positively charged carbon atom, are pivotal in countless organic reactions. Understanding their stability is crucial for predicting reaction pathways and yields. Of the various types of carbocations (methyl, primary, secondary, and tertiary), tertiary carbocations are significantly more stable. This article will delve into the reasons behind this enhanced stability, exploring the key factors contributing to it and providing illustrative examples.
The Fundamentals: Carbocation Structure and Stability
Before diving into the specifics of tertiary carbocation stability, let's establish a basic understanding. A carbocation is a carbon atom with only three bonds and a positive formal charge. This positive charge signifies a deficiency of electrons, making carbocations highly reactive electrophilic species. Their stability is inversely proportional to their reactivity; a more stable carbocation is less reactive.
The stability of a carbocation is primarily determined by the electron-donating ability of the groups attached to the positively charged carbon. This electron donation helps to delocalize the positive charge, effectively reducing its magnitude and thus increasing the carbocation's stability.
Why Tertiary Carbocations Reign Supreme: The Impact of Alkyl Groups
The key to understanding the superior stability of tertiary carbocations lies in the inductive effect and hyperconjugation. Let's explore both:
The Inductive Effect: Electron Donation Through Sigma Bonds
Alkyl groups (like methyl, ethyl, propyl) are electron-donating groups due to the inductive effect. This effect arises from the difference in electronegativity between carbon and hydrogen. Carbon is slightly less electronegative than hydrogen, meaning it holds the shared electrons in a C-H bond slightly less tightly. Consequently, when an alkyl group is attached to a positively charged carbon, it pushes electron density towards the positive charge, thereby stabilizing it.
The more alkyl groups attached, the greater the inductive effect. A tertiary carbocation benefits from three alkyl groups donating electron density, significantly reducing the positive charge density on the central carbon. In contrast, a primary carbocation only has one alkyl group offering this stabilizing inductive effect, while a secondary carbocation has two. This directly correlates with the observed stability order: tertiary > secondary > primary > methyl.
Hyperconjugation: Electron Delocalization Through Overlap of Orbitals
Hyperconjugation is a crucial stabilizing factor, particularly significant in carbocations. It involves the interaction between the empty p-orbital of the positively charged carbon and the adjacent C-H sigma bonding orbitals. This interaction allows for delocalization of electron density from the C-H sigma bond into the empty p-orbital, effectively spreading the positive charge over a larger area.
The number of adjacent C-H bonds directly impacts the extent of hyperconjugation. A tertiary carbocation possesses a maximum number of adjacent C-H bonds, allowing for greater hyperconjugation and consequently enhanced stability. This explains the significant stability difference between tertiary and secondary or primary carbocations, where the number of adjacent C-H bonds is lower.
Visualizing Hyperconjugation: Imagine the C-H bond electrons as slightly shifting towards the positively charged carbon, creating a partial double bond character between the carbon and the adjacent carbon atom. This effectively reduces the positive charge on the carbocation.
Comparing Stability: A Detailed Look at Carbocation Types
Let's directly compare the stability of different carbocations, emphasizing the roles of inductive effect and hyperconjugation:
-
Methyl Carbocation (CH3+): This carbocation is the least stable. It has no alkyl groups to donate electron density through the inductive effect, and the hyperconjugation is minimal due to the limited number of adjacent C-H bonds. The positive charge is highly concentrated on the central carbon, making it extremely reactive.
-
Primary Carbocation (RCH2+): A primary carbocation benefits from the inductive effect of a single alkyl group, offering some stabilization. Hyperconjugation is also present but limited compared to higher-order carbocations. It's more stable than a methyl carbocation but significantly less stable than secondary and tertiary counterparts.
-
Secondary Carbocation (R2CH+): A secondary carbocation benefits from the inductive effect of two alkyl groups, resulting in more electron density donation towards the positive charge. The hyperconjugation is also more extensive than in primary carbocations, leading to greater stability compared to primary carbocations.
-
Tertiary Carbocation (R3C+): The tertiary carbocation is the most stable due to the combined effects of the inductive effect from three alkyl groups and the maximum possible hyperconjugation. The positive charge is significantly delocalized across the molecule, leading to reduced reactivity and enhanced stability.
Experimental Evidence and Applications
The enhanced stability of tertiary carbocations is not just a theoretical concept; it's supported by numerous experimental observations. For instance, the relative rates of SN1 reactions (nucleophilic substitution reactions proceeding via a carbocation intermediate) clearly reflect the stability order. Tertiary substrates undergo SN1 reactions much faster than primary or secondary substrates, directly correlating with the greater stability of the intermediate tertiary carbocation.
The understanding of carbocation stability is crucial in many areas of organic chemistry, including:
- Predicting reaction mechanisms: Knowing the relative stability of carbocations helps in predicting whether a reaction will proceed via a carbocation intermediate and the likely pathway.
- Designing organic syntheses: Chemists utilize this knowledge to design synthetic routes that favor the formation of more stable carbocations, enhancing reaction efficiency and yield.
- Understanding biological processes: Carbocation intermediates are involved in various biological processes, and their stability is a crucial factor influencing reaction rates and selectivity in enzymatic reactions.
Beyond Alkyl Groups: Other Factors Influencing Carbocation Stability
While alkyl groups are the primary contributors to carbocation stability, other factors can also play a role:
-
Resonance: Carbocations that can participate in resonance stabilization (where the positive charge can be delocalized through a pi system) are exceptionally stable. Allylic and benzylic carbocations are excellent examples, benefiting from both inductive effects and resonance.
-
Solvent Effects: The solvent environment can also influence carbocation stability. Polar solvents, capable of stabilizing the positive charge through solvation, can enhance the stability of carbocations.
Conclusion: The Paramount Importance of Tertiary Carbocation Stability
The enhanced stability of tertiary carbocations arises from the synergistic effects of the inductive effect and hyperconjugation. These effects effectively delocalize the positive charge, reducing its magnitude and making the carbocation less reactive. This understanding is not only fundamental to understanding organic reaction mechanisms but also critical for designing effective and efficient synthetic strategies in various chemical and biological contexts. The superior stability of tertiary carbocations compared to other carbocation types highlights the powerful impact of alkyl group substitution and electron delocalization in stabilizing reactive intermediates. This knowledge serves as a cornerstone in the field of organic chemistry.
Latest Posts
Latest Posts
-
Surface Integral Of A Sphere In Spherical Coordinates
Mar 29, 2025
-
Report Sheet Chemical Reactions Experiment 4
Mar 29, 2025
-
What Makes A Good Recrystallization Solvent
Mar 29, 2025
-
Mannitol Salt Agar Is Selective For Which Bacterial Genus
Mar 29, 2025
-
How To Do Statistical Data Transformations In Excel
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Why Are Tertiary Carbocations More Stable . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
