Why Are Unsaturated Fats Liquid At Room Temperature
Muz Play
Apr 06, 2025 · 5 min read
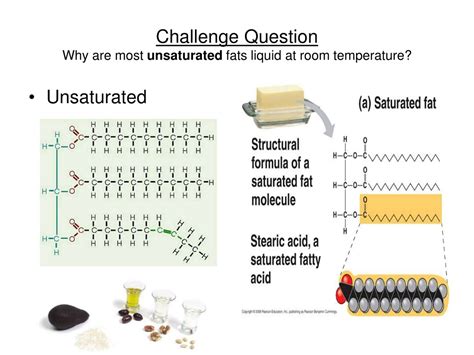
Table of Contents
Why Are Unsaturated Fats Liquid at Room Temperature? A Deep Dive into Fatty Acid Structure and Properties
Unsaturated fats, a cornerstone of a healthy diet, are known for their liquid state at room temperature. This contrasts sharply with saturated fats, which are typically solid. But why this crucial difference? The answer lies in the molecular structure of the fatty acids that comprise these fats. Understanding this difference is key to appreciating the impact of dietary fats on our health and overall well-being. This comprehensive article delves into the chemistry behind unsaturated fats' fluidity, exploring the types of unsaturation, their impact on molecular packing, and the implications for human health.
The Role of Fatty Acids: The Building Blocks of Fats
Fats, or triglycerides, are composed of three fatty acids attached to a glycerol molecule. Fatty acids are long chains of carbon atoms, with a carboxyl group (-COOH) at one end. It's the structure of these fatty acid chains that dictates whether a fat will be solid or liquid at room temperature. This structure is characterized by the presence or absence of double bonds between carbon atoms.
Saturated vs. Unsaturated: The Key Distinction
-
Saturated fatty acids: These fatty acids have no double bonds between carbon atoms. Each carbon atom is "saturated" with hydrogen atoms. This allows the molecules to pack tightly together, resulting in a solid structure at room temperature. Think of it like neatly stacked pencils – they occupy minimal space. Examples include the fatty acids found in butter and lard.
-
Unsaturated fatty acids: These fatty acids have one or more double bonds between carbon atoms. The presence of these double bonds introduces "kinks" or "bends" in the fatty acid chain, preventing them from packing together as tightly as saturated fatty acids. This loose packing results in a liquid state at room temperature. Imagine trying to stack bent pencils – it's much harder and they take up more space. This is analogous to the behavior of unsaturated fats.
Types of Unsaturated Fats: Monounsaturated and Polyunsaturated
Unsaturated fats are further categorized based on the number of double bonds present:
Monounsaturated Fats: One Double Bond
Monounsaturated fats contain only one double bond in their fatty acid chains. This single double bond creates a single bend in the molecule, making packing less efficient compared to saturated fats, but still relatively ordered compared to polyunsaturated fats. Olive oil is a rich source of monounsaturated fats, specifically oleic acid. The presence of this single double bond contributes to olive oil's liquid state at room temperature.
Polyunsaturated Fats: Multiple Double Bonds
Polyunsaturated fats contain two or more double bonds in their fatty acid chains. These multiple double bonds introduce multiple bends and kinks, significantly disrupting the ability of the molecules to pack together. This leads to a more fluid state, even at lower temperatures than monounsaturated fats. Examples include linoleic acid (omega-6) and alpha-linolenic acid (omega-3), which are essential fatty acids found in vegetable oils like soybean oil, sunflower oil, and flaxseed oil. The multiple kinks prevent the formation of a solid structure, hence their liquid nature at room temperature.
Cis vs. Trans Unsaturated Fats: A Crucial Difference
The configuration of the double bonds also plays a significant role in the properties of unsaturated fats. Double bonds can exist in two configurations:
-
Cis configuration: In cis unsaturated fats, the hydrogen atoms attached to the carbon atoms involved in the double bond are on the same side of the double bond. This creates a significant bend in the fatty acid chain. Most naturally occurring unsaturated fats are in the cis configuration.
-
Trans configuration: In trans unsaturated fats, the hydrogen atoms attached to the carbon atoms involved in the double bond are on opposite sides of the double bond. This results in a straighter fatty acid chain, allowing for slightly tighter packing than cis unsaturated fats. Trans fats are primarily produced artificially during the process of partial hydrogenation of vegetable oils. This process straightens out some of the cis double bonds, creating trans fats. Trans fats are associated with negative health effects and are largely avoided in modern diets.
The Impact of Double Bonds on Melting Point
The presence and configuration of double bonds directly affect the melting point of fats. The more double bonds present, and the more they are in the cis configuration, the lower the melting point. This is because the kinks and bends prevent efficient molecular packing, requiring less energy to overcome the intermolecular forces holding the molecules together. This explains why unsaturated fats remain liquid at room temperature, whereas saturated fats with their tightly packed structures have higher melting points and are solid at room temperature.
The Significance of Unsaturated Fats in Human Health
The fluidity of unsaturated fats is not merely a chemical curiosity; it has profound implications for human health. Unsaturated fats are crucial components of cell membranes, influencing their fluidity and permeability. They are also precursors to important signaling molecules called eicosanoids, involved in inflammation and blood clotting.
-
Omega-3 Fatty Acids: These polyunsaturated fats are particularly important for heart health, reducing the risk of heart disease and stroke. They help lower blood pressure, triglycerides, and inflammation.
-
Omega-6 Fatty Acids: While essential, omega-6 fatty acids need to be balanced with omega-3s. An imbalance favoring omega-6 can contribute to inflammation.
-
Monounsaturated Fats: These fats also contribute to heart health and can help improve cholesterol levels.
Conclusion: Fluidity, Structure, and Health
The liquid state of unsaturated fats at room temperature is a direct consequence of their chemical structure. The presence of double bonds in the fatty acid chains introduces kinks and bends, preventing the molecules from packing tightly together. This loose packing results in a lower melting point and a liquid state at room temperature. Understanding this fundamental relationship between molecular structure and physical properties is crucial for appreciating the role of unsaturated fats in our diet and their impact on our overall health. A balanced intake of unsaturated fats, with an appropriate ratio of omega-3 to omega-6 fatty acids, is essential for maintaining optimal health and well-being. By understanding the chemistry behind their fluidity, we can make more informed choices about the fats we consume and contribute to a healthier lifestyle. Remember to always consult with a healthcare professional or registered dietitian for personalized dietary advice.
Latest Posts
Latest Posts
-
Do All Cells Come From Preexisting Cells
Apr 08, 2025
-
Where Do The Electrons Entering Photosystem Ii Come From
Apr 08, 2025
-
Why Is A Cell A Basic Unit Of Life
Apr 08, 2025
-
How To Calculate Average Molecular Speed
Apr 08, 2025
-
How To Find A Limit On A Graph
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Why Are Unsaturated Fats Liquid At Room Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.