Why Can't The Subscripts Be Changed In A Chemical Equation
Muz Play
Apr 04, 2025 · 5 min read
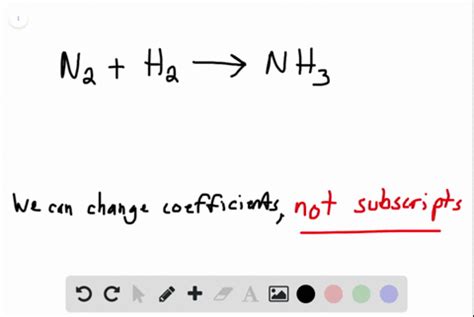
Table of Contents
Why Can't the Subscripts Be Changed in a Chemical Equation? The Importance of Stoichiometry
Chemical equations are the bedrock of chemistry, representing the symbolic shorthand for chemical reactions. They tell us what reactants are consumed and what products are formed, and crucially, in what quantities. A fundamental aspect of these equations is the use of subscripts to denote the number of atoms of each element within a molecule. These subscripts are not arbitrary; they are dictated by the fundamental laws of chemistry and cannot be altered without fundamentally changing the nature of the substances involved. Changing subscripts in a chemical equation would violate the law of conservation of mass and alter the chemical identities of the substances, rendering the equation incorrect and meaningless. Let's delve into the reasons behind this immutable rule.
The Unbreakable Law of Conservation of Mass
At the heart of this matter lies the law of conservation of mass, a cornerstone principle in chemistry. This law states that matter cannot be created or destroyed in a chemical reaction; it simply changes form. The total mass of the reactants must equal the total mass of the products. This principle directly impacts the subscripts in a chemical equation.
Subscripts Represent Atomic Ratios
Subscripts in a chemical formula represent the ratio of atoms of each element present in a molecule or compound. For example, in the formula H₂O (water), the subscript '2' indicates that there are two hydrogen atoms for every one oxygen atom. This fixed ratio is crucial to the properties and identity of water. Changing the subscript would create a different molecule altogether. If we were to change the subscript to H₂O₂, we would no longer have water; instead, we'd have hydrogen peroxide, a completely different substance with different chemical and physical properties.
Balancing Equations vs. Changing Subscripts
It's important to distinguish between balancing a chemical equation and changing subscripts. Balancing an equation involves adjusting the coefficients (the numbers placed in front of the chemical formulas) to ensure that the number of atoms of each element is the same on both sides of the equation. This process maintains the integrity of the chemical formulas and respects the law of conservation of mass.
For instance, consider the reaction between hydrogen and oxygen to form water:
H₂ + O₂ → H₂O
This equation is unbalanced because there are two oxygen atoms on the left but only one on the right. To balance it, we adjust the coefficients:
2H₂ + O₂ → 2H₂O
Now, there are four hydrogen atoms and two oxygen atoms on both sides, satisfying the law of conservation of mass. Crucially, the subscripts within the chemical formulas (H₂ and O₂) remain unchanged. Changing them would violate the fundamental composition of the molecules involved.
Chemical Identity and Subscripts
The subscripts in a chemical formula are inextricably linked to the chemical identity of the substance. Each element has specific chemical properties, and the way atoms are bonded together determines the overall characteristics of the compound. Changing the subscript directly alters this bonding arrangement, thereby creating a different substance.
Example: Carbon Monoxide vs. Carbon Dioxide
Consider carbon monoxide (CO) and carbon dioxide (CO₂). These are two distinct gases with vastly different properties. CO is toxic, while CO₂ is essential for plant life. The difference boils down to the single oxygen atom difference; the subscript of oxygen in the formula dictates the identity and characteristics of the molecule. Altering the subscript would transform one molecule into another, leading to a false representation of the chemical reaction.
Implications of Incorrect Subscripts
Using incorrect subscripts would have serious consequences, especially in practical applications:
-
Incorrect Stoichiometric Calculations: Chemical reactions are governed by precise stoichiometric ratios. Incorrect subscripts would lead to inaccurate calculations of reactant and product quantities, potentially leading to hazardous or inefficient processes in industrial chemistry, pharmaceutical manufacturing, or environmental remediation.
-
Misinterpretation of Chemical Reactions: Incorrect subscripts would lead to a flawed understanding of the reaction mechanism and the products formed. This would hinder scientific progress and innovation.
-
Safety Hazards: In experimental settings or industrial processes, using incorrect subscripts could lead to unintended reactions, producing unexpected and potentially dangerous substances. This could endanger personnel and damage equipment.
The Role of Chemical Bonding
The subscripts are also directly linked to the type of chemical bonding present. The arrangement of atoms, determined by the subscripts, dictates how electrons are shared or transferred between atoms, shaping the molecule's structure, properties, and reactivity. A change in subscripts fundamentally alters the bonding pattern.
Covalent vs. Ionic Bonding
In covalent compounds, atoms share electrons; the subscripts reflect the number of atoms participating in the sharing. In ionic compounds, atoms transfer electrons, and subscripts indicate the ratio of ions needed to balance the charges. In both cases, altering the subscripts disrupts the fundamental bonding arrangement and creates a different substance.
Beyond the Basics: Polyatomic Ions and Complex Compounds
The importance of unchanging subscripts extends beyond simple molecules to more complex chemical entities like polyatomic ions and coordination compounds. Polyatomic ions, such as sulfate (SO₄²⁻) or nitrate (NO₃⁻), have fixed subscripts defining their composition and charge. Changing these subscripts alters the ion's identity and charge, which would impact the overall charge balance in the chemical equation and invalidate the entire representation of the reaction. Similar considerations apply to coordination compounds, where ligands bind to a central metal atom in specific numbers, indicated by subscripts. Altering these would disrupt the coordination sphere and create a different complex.
Conclusion: The Inviolable Nature of Subscripts
In conclusion, the subscripts in a chemical equation cannot be changed because they represent the fundamental composition and identity of the molecules involved. Changing them would violate the law of conservation of mass, alter the chemical identities of the substances, and lead to incorrect and potentially dangerous interpretations of chemical reactions. The subscripts are not arbitrary; they are dictated by the intricate rules of chemical bonding, stoichiometry, and the immutable laws of nature governing chemical transformations. Respecting the fixed nature of subscripts is paramount for accurate representation, proper stoichiometric calculations, and ensuring safety and efficiency in all chemical applications. The understanding and proper application of this fundamental principle are vital for any student or professional working in the field of chemistry.
Latest Posts
Latest Posts
-
Is This Bacterial Isolate Positive Or Negative For Catalase Activity
Apr 10, 2025
-
What Sublevels Are Filling Across The Transition Metals
Apr 10, 2025
-
S P D And F Blocks On The Periodic Table
Apr 10, 2025
-
Which Term Represents A Phase Change
Apr 10, 2025
-
How To Draw Bond Line Structures
Apr 10, 2025
Related Post
Thank you for visiting our website which covers about Why Can't The Subscripts Be Changed In A Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.