Why Do Covalent Compounds Have Low Melting Points
Muz Play
Apr 06, 2025 · 6 min read
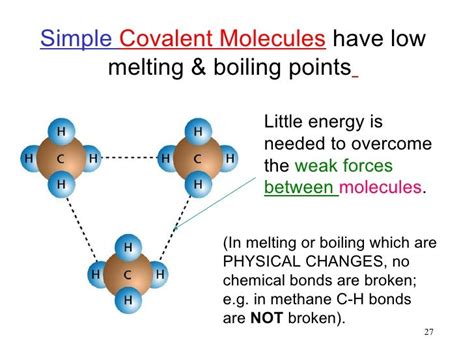
Table of Contents
Why Do Covalent Compounds Have Low Melting Points?
Covalent compounds, characterized by the sharing of electrons between atoms to form strong covalent bonds, exhibit a wide range of melting points. While some covalent compounds have exceptionally high melting points, many possess significantly lower melting points compared to ionic compounds. Understanding this disparity requires exploring the fundamental nature of covalent bonding and the intermolecular forces that govern the physical properties of these substances. This article will delve into the reasons behind the relatively low melting points observed in many covalent compounds.
The Nature of Covalent Bonds
Before examining melting points, it's crucial to understand the nature of covalent bonds themselves. Covalent bonds arise from the mutual sharing of electron pairs between atoms. This sharing results in a stable, relatively strong bond within the molecule. The strength of these bonds is dependent on factors like the electronegativity difference between the atoms involved and the number of shared electron pairs (single, double, or triple bonds). The strength of the intramolecular covalent bonds is NOT directly responsible for the low melting point, however.
Intramolecular vs. Intermolecular Forces
The key to understanding the melting point lies in the distinction between intramolecular forces (forces within the molecule) and intermolecular forces (forces between molecules). While covalent bonds are strong intramolecular forces, the forces holding different molecules together are relatively weaker intermolecular forces. It's the intermolecular forces, not the strong covalent bonds, that determine the melting point of a covalent compound.
Intermolecular Forces and Their Impact on Melting Point
Melting a solid involves overcoming the intermolecular forces holding the molecules together in a fixed, ordered arrangement. The weaker these intermolecular forces, the less energy is required to break them, resulting in a lower melting point. Several types of intermolecular forces exist, including:
1. London Dispersion Forces (LDFs): The Universal Force
London Dispersion Forces (LDFs), also known as van der Waals forces, are the weakest type of intermolecular force. They arise from temporary fluctuations in electron distribution around an atom or molecule, creating temporary dipoles. These temporary dipoles induce dipoles in neighboring molecules, leading to weak attractive forces. LDFs exist between all molecules, regardless of polarity. The strength of LDFs increases with the size and surface area of the molecule. Larger molecules have more electrons, leading to larger temporary dipoles and stronger LDFs.
2. Dipole-Dipole Forces: Polarity Matters
Dipole-dipole forces occur between polar molecules, those with a permanent dipole moment due to an uneven distribution of electron density. The positive end of one polar molecule attracts the negative end of another, resulting in a stronger attraction than LDFs. The strength of dipole-dipole forces is greater than LDFs but still significantly weaker than covalent bonds.
3. Hydrogen Bonding: A Special Case
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to a lone pair of electrons on another electronegative atom in a nearby molecule. Hydrogen bonds are stronger than typical dipole-dipole forces but still considerably weaker than covalent bonds. Water (H₂O) is a prime example of a molecule exhibiting strong hydrogen bonding, leading to its relatively high boiling point compared to other similar-sized molecules.
4. Ion-Dipole Forces (Relevant in certain covalent compound scenarios)
While less common in purely covalent scenarios, ion-dipole forces become relevant when a covalent compound dissolves in a polar solvent. The charged ions from a dissolved salt will interact with the dipole of the covalent molecules, influencing physical properties like melting point in solutions. This is often not the primary reason for low melting points in pure covalent compounds.
Why Covalent Compounds Often Have Low Melting Points: A Summary
Many covalent compounds have low melting points because the intermolecular forces holding their molecules together are relatively weak. These weak intermolecular forces (primarily LDFs, with sometimes dipole-dipole forces and hydrogen bonding playing a role) require relatively little energy to overcome during melting. This contrasts sharply with ionic compounds, where strong electrostatic attractions between oppositely charged ions require substantially more energy to break.
Let's illustrate this with some examples:
-
Simple Molecular Substances: Molecules like methane (CH₄) and carbon dioxide (CO₂) are held together primarily by weak LDFs. Therefore, these compounds have very low melting points.
-
Larger Organic Molecules: Larger organic molecules like hydrocarbons also exhibit low melting points due to increased LDFs that, while stronger than those in smaller molecules, are still far weaker than ionic bonds. The increased size also leads to increased degrees of freedom, reducing the efficiency of packing and thus the intermolecular forces.
-
Network Covalent Compounds (Exception): It's crucial to note that some covalent compounds, such as diamond and quartz (silicon dioxide), have exceptionally high melting points. This is because they form giant covalent structures with a continuous network of strong covalent bonds throughout the entire solid. The high energy required to break this vast network of covalent bonds results in their high melting points. These are exceptions to the general trend.
Factors Affecting the Melting Point of Covalent Compounds
Several factors influence the melting point of covalent compounds beyond the simple presence of intermolecular forces:
-
Molecular Size and Shape: Larger molecules generally have higher melting points due to stronger LDFs. Molecular shape also plays a role, as more compact molecules pack more efficiently, leading to stronger intermolecular interactions.
-
Polarity: Polar molecules with dipole-dipole forces or hydrogen bonds have higher melting points than nonpolar molecules with only LDFs.
-
Branching: Branching in organic molecules can reduce the efficiency of packing, leading to weaker intermolecular forces and lower melting points. Straight-chain molecules pack more efficiently than branched molecules.
-
Symmetry: Symmetrical molecules often pack more efficiently than asymmetrical molecules, leading to stronger intermolecular forces and higher melting points.
Comparing Covalent and Ionic Compounds: A Key Distinction
The difference in melting points between covalent and ionic compounds stems from the fundamental difference in bonding. Ionic compounds are characterized by strong electrostatic attractions between oppositely charged ions, forming a rigid lattice structure. Overcoming these strong forces requires significant energy, resulting in high melting points. Covalent compounds, on the other hand, are held together by weaker intermolecular forces, requiring less energy to overcome during melting, hence their relatively lower melting points.
Conclusion
The relatively low melting points of many covalent compounds are directly related to the weak nature of the intermolecular forces that hold their molecules together. While the intramolecular covalent bonds are strong, they do not govern the melting process. The strength of LDFs, dipole-dipole forces, and hydrogen bonds significantly influence the energy required to transition from solid to liquid. Larger molecules, branched molecules, and molecules with less efficient packing tend to have lower melting points. Understanding these relationships is crucial for predicting and interpreting the physical properties of a wide variety of covalent compounds. While network covalent compounds serve as exceptions to the low melting point rule, they underscore the importance of considering the overall structure and bonding in determining physical properties.
Latest Posts
Latest Posts
-
What Is The Difference Between Inequality And Equation
Apr 08, 2025
-
The Change Of State From Liquid To Gas Is Called
Apr 08, 2025
-
Every Joke Has A Grain Of Truth
Apr 08, 2025
-
What Do Elements In A Period Have In Common
Apr 08, 2025
-
Can A Pure Substance Be Broken Down
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Why Do Covalent Compounds Have Low Melting Points . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.