Why Do Ionic Compounds Have High Melting And Boiling Points
Muz Play
Mar 27, 2025 · 6 min read
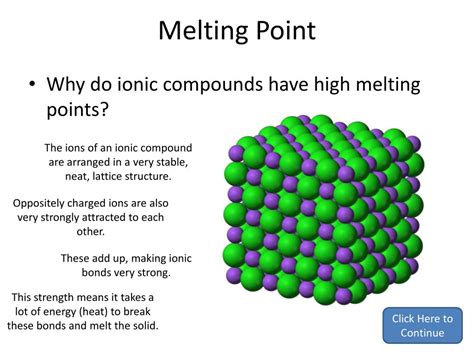
Table of Contents
Why Do Ionic Compounds Have High Melting and Boiling Points?
Ionic compounds are known for their remarkably high melting and boiling points compared to other types of compounds like covalent compounds. Understanding this characteristic requires delving into the fundamental nature of ionic bonds and the strong forces that govern their interactions. This article will explore the reasons behind this phenomenon, examining the electrostatic forces, lattice energy, and factors influencing melting and boiling points in detail.
The Strong Grip of Electrostatic Attraction: The Ionic Bond
At the heart of this high melting and boiling point characteristic lies the ionic bond, a powerful electrostatic attraction between oppositely charged ions. Unlike covalent compounds, where atoms share electrons, ionic compounds involve the transfer of electrons from one atom to another. This transfer creates ions: positively charged cations (usually metals) and negatively charged anions (usually non-metals). The intense electrostatic attraction between these ions is the driving force behind the strong cohesive forces within the ionic lattice.
Cations and Anions: A Dance of Opposites
The formation of ions is crucial. Metals, with relatively low electronegativity, tend to lose electrons, forming positively charged cations. Non-metals, with higher electronegativity, readily gain electrons to become negatively charged anions. The difference in electronegativity between the metal and non-metal is a key factor in determining the strength of the ionic bond. A larger difference leads to a stronger bond.
The Ionic Lattice: A Crystalline Structure
These ions don't exist in isolation. They arrange themselves in a highly ordered, three-dimensional structure known as an ionic lattice. This lattice is a repeating pattern of alternating cations and anions, maximizing electrostatic attraction and minimizing repulsion. The specific arrangement depends on the size and charge of the ions involved. The overall neutrality of the lattice is maintained because the total positive charge of the cations equals the total negative charge of the anions.
Lattice Energy: The Measure of Ionic Bond Strength
The energy required to completely separate one mole of a solid ionic compound into its gaseous ions is called lattice energy. It's a direct measure of the strength of the ionic bonds within the lattice. High lattice energy signifies strong ionic bonds, and this directly correlates with high melting and boiling points. Several factors influence lattice energy:
Charge of Ions: The Magnitude Matters
The magnitude of the charges on the ions significantly impacts lattice energy. Greater charges lead to stronger electrostatic attractions and, therefore, higher lattice energy. For example, the lattice energy of magnesium oxide (MgO), with Mg²⁺ and O²⁻ ions, is much higher than that of sodium chloride (NaCl), with Na⁺ and Cl⁻ ions. This difference in charge accounts for the much higher melting and boiling points of MgO compared to NaCl.
Size of Ions: Distance Makes a Difference
The distance between the ions also plays a critical role. Smaller ions are closer together, resulting in stronger electrostatic attractions and higher lattice energy. Larger ions are further apart, experiencing weaker attractions and lower lattice energy. This is why compounds with smaller ions generally have higher melting and boiling points than those with larger ions.
The Combined Effect: Charge and Size
The combined effect of charge and size is crucial. A compound with highly charged, small ions will have exceptionally high lattice energy and consequently, very high melting and boiling points. Conversely, a compound with low-charged, large ions will exhibit lower lattice energy and lower melting and boiling points.
Overcoming the Lattice: Melting and Boiling
To melt an ionic compound, you must overcome the strong electrostatic forces holding the ions in the lattice. This requires sufficient energy to disrupt the ordered arrangement and allow the ions to move more freely. Similarly, boiling involves completely overcoming these forces to convert the solid into a gas, where ions are essentially independent of each other.
The Energy Barrier
The high melting and boiling points of ionic compounds are a direct consequence of the substantial energy needed to break the strong ionic bonds and overcome the lattice energy. The stronger the ionic bonds (higher lattice energy), the more energy is required, resulting in higher melting and boiling points.
Factors Affecting Melting and Boiling Points Beyond Lattice Energy
While lattice energy is the dominant factor, other factors can subtly influence the melting and boiling points of ionic compounds:
Polarizability of Ions: A Secondary Effect
The polarizability of ions, their ability to have their electron clouds distorted, can slightly influence the strength of interactions. More polarizable ions can experience temporary, induced dipole-dipole interactions, adding a small contribution to the overall attraction.
Crystal Structure: Arrangement Matters
The specific crystal structure of the ionic compound, the geometric arrangement of ions in the lattice, also plays a minor role. Different structures can have slightly different packing efficiencies and thus influence the strength of overall lattice interactions.
Covalent Character: A Complicating Factor
In some ionic compounds, particularly those involving transition metals, there might be a degree of covalent character in the bonding. This partial sharing of electrons can slightly reduce the purely ionic character and subtly affect the melting and boiling points. This is less pronounced in simple ionic compounds formed by alkali metals and halogens.
Examples and Comparisons
Let's consider some examples to illustrate these principles:
-
Sodium Chloride (NaCl): A relatively simple ionic compound with a relatively low charge (+1 and -1) and moderately sized ions. It has a high melting point (801 °C) and boiling point (1413 °C), demonstrating the significant energy required to break the ionic bonds.
-
Magnesium Oxide (MgO): This compound has higher charges (+2 and -2) and smaller ions than NaCl. This results in much stronger electrostatic attractions and a considerably higher melting point (2852 °C) and boiling point (3600 °C).
-
Aluminum Oxide (Al₂O₃): With even higher charges (+3 and -2) and relatively small ions, aluminum oxide boasts an extremely high melting point (2072 °C) and boiling point (2977 °C), showcasing the dramatic effect of increased ionic charge on melting and boiling points.
Conclusion: A Strong Foundation of Electrostatic Attraction
The high melting and boiling points of ionic compounds are a direct consequence of the strong electrostatic attraction between oppositely charged ions in the ionic lattice. The lattice energy, determined by the charge and size of the ions, is the primary factor governing this property. While other factors such as polarizability and crystal structure play a secondary role, the fundamental principle remains: the stronger the ionic bonds, the higher the energy needed to disrupt the lattice, leading to exceptionally high melting and boiling points. Understanding this relationship is crucial in predicting and explaining the properties of ionic compounds in various chemical and physical contexts.
Latest Posts
Latest Posts
-
Identification Of Unknown Bacteria Lab Report Pdf
Mar 30, 2025
-
Difference Between The Autonomic And Somatic Nervous System
Mar 30, 2025
-
Examples Of Literary Analysis Thesis Statements
Mar 30, 2025
-
Why Might Two Elements Possess Similar Chemical Properties
Mar 30, 2025
-
Where Does Water Enter The Plant
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Why Do Ionic Compounds Have High Melting And Boiling Points . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
