Why Does Km Increase In Competitive Inhibition
Muz Play
Mar 31, 2025 · 5 min read
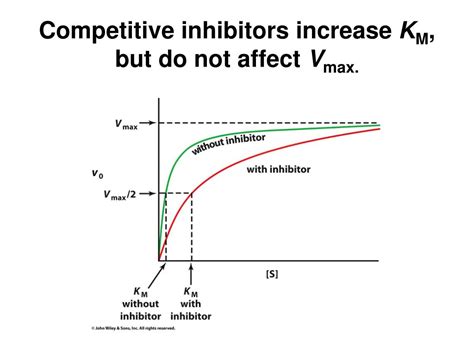
Table of Contents
Why Does Km Increase in Competitive Inhibition? A Deep Dive into Enzyme Kinetics
Enzyme kinetics is a cornerstone of biochemistry, providing crucial insights into enzyme function and regulation. Competitive inhibition, a common form of enzyme regulation, presents a fascinating case study in understanding enzyme-substrate interactions. A key characteristic of competitive inhibition is the increase in the Michaelis-Menten constant (Km). But why does this happen? This article will delve deep into the mechanistic underpinnings of competitive inhibition, explaining the increase in Km and its implications.
Understanding the Michaelis-Menten Kinetics
Before diving into competitive inhibition, it's crucial to grasp the fundamentals of Michaelis-Menten kinetics. This model describes the relationship between the initial reaction velocity (V₀) of an enzyme-catalyzed reaction and the substrate concentration ([S]). The equation is:
V₀ = (Vmax [S]) / (Km + [S])
Where:
- Vmax: The maximum reaction velocity achieved when the enzyme is saturated with substrate.
- Km: The Michaelis constant, representing the substrate concentration at which the reaction velocity is half of Vmax. Km is a measure of the enzyme's affinity for its substrate; a lower Km indicates higher affinity.
The Mechanism of Competitive Inhibition
Competitive inhibitors resemble the substrate and compete for binding to the enzyme's active site. This competition is the defining characteristic of competitive inhibition. The inhibitor (I) binds reversibly to the active site, preventing substrate binding. Crucially, the inhibitor does not alter the enzyme's catalytic activity. If the inhibitor is bound, no reaction can occur; only when the substrate binds can catalysis proceed.
Visualizing the Competition
Imagine the active site as a lock and the substrate as the key that fits perfectly, initiating the reaction (opening the lock). A competitive inhibitor is like a similar-shaped key that also tries to fit into the lock. While it might fit somewhat, it doesn't turn, preventing the "real" key (substrate) from accessing the lock. The more "fake keys" (inhibitor) present, the harder it is for the "real key" to get in.
The Impact on Km: Why It Increases
The presence of a competitive inhibitor affects the apparent Km of the enzyme. This is because the inhibitor effectively reduces the number of active enzyme molecules available to bind the substrate. To reach half of the maximum velocity (Vmax) in the presence of the inhibitor, a higher substrate concentration is now required.
This is why Km increases in competitive inhibition. The apparent Km (Km<sub>app</sub>) in the presence of a competitive inhibitor is given by:
Km<sub>app</sub> = Km (1 + [I]/Ki)
Where:
- [I]: The concentration of the inhibitor.
- Ki: The inhibitor dissociation constant, representing the inhibitor's affinity for the enzyme; a lower Ki indicates higher affinity.
This equation clearly shows that as the inhibitor concentration ([I]) increases, the apparent Km (Km<sub>app</sub>) also increases. The higher the inhibitor concentration, the more substrate is needed to achieve half the maximum velocity.
A Detailed Explanation of the Increase
Let's break down why this mathematical relationship reflects the reality of the enzyme-substrate interaction:
-
Reduced Active Sites: The competitive inhibitor occupies the active site, effectively reducing the number of active sites available for substrate binding. This makes it harder for the substrate to bind to the enzyme.
-
Increased Substrate Needed: To overcome this reduced availability of active sites, a higher substrate concentration is required to maintain a given reaction rate. In essence, the enzyme needs "more substrate to compensate for the inhibitor".
-
Apparent Affinity Decrease: The increase in Km reflects a seeming decrease in the enzyme's affinity for the substrate. The enzyme is still just as capable of binding the substrate if the active site is available, but the inhibitor makes the active site availability much lower.
-
Vmax Remains Unchanged: It's crucial to note that while Km increases, Vmax remains unchanged in competitive inhibition. If enough substrate is added, it can outcompete the inhibitor and reach the same maximum velocity as in the absence of the inhibitor. This is because the inhibitor does not affect the enzyme's catalytic function once the substrate has bound successfully.
Experimental Evidence and Lineweaver-Burk Plots
The increase in Km in competitive inhibition can be experimentally demonstrated using techniques like Lineweaver-Burk plots. These plots are double reciprocal plots of the Michaelis-Menten equation (1/V₀ vs 1/[S]). In the presence of a competitive inhibitor:
- The y-intercept (1/Vmax) remains unchanged.
- The x-intercept (-1/Km) shifts to the right, indicating an increased Km.
- The lines intersect on the y-axis.
This graphical representation provides compelling experimental support for the increase in Km.
Examples of Competitive Inhibition in Biological Systems
Competitive inhibition plays a crucial role in various biological processes. Many drugs function as competitive inhibitors, targeting specific enzymes to treat various diseases. For example:
- Methotrexate: A competitive inhibitor of dihydrofolate reductase, an enzyme involved in nucleotide synthesis. It is used in cancer chemotherapy.
- Sulfonamides: Competitive inhibitors of para-aminobenzoic acid (PABA) synthesis, essential for bacterial growth. They are used as antibacterial agents.
- Statins: Competitive inhibitors of HMG-CoA reductase, an enzyme involved in cholesterol biosynthesis. They lower cholesterol levels.
These examples underscore the significant biological implications of competitive inhibition and the importance of understanding its effects on enzyme kinetics.
Implications and Conclusion
The increase in Km in competitive inhibition is a fundamental aspect of enzyme kinetics with significant implications in various fields, including drug discovery and metabolic regulation. Understanding this phenomenon is crucial for designing effective drugs, understanding metabolic pathways, and developing strategies for regulating enzyme activity. The unchanging Vmax, in contrast to the altered Km, provides a diagnostic tool for identifying competitive inhibition experimentally. By appreciating the molecular mechanisms underlying this type of inhibition, we gain a deeper understanding of how enzyme activity is modulated within the complex milieu of the cell. The mathematical description, complemented by experimental techniques like the Lineweaver-Burk plot, offers a powerful framework for interpreting and predicting the behavior of enzymes under competitive inhibition. This foundational knowledge is critical for advancements in areas such as drug design, metabolic engineering, and fundamental biochemical research.
Latest Posts
Latest Posts
-
Which Of The Following Situations Will Lead To Natural Selection
Apr 01, 2025
-
How Does The Nucleus And Ribosomes Work Together
Apr 01, 2025
-
Having A Single Set Of Unpaired Chromosomes
Apr 01, 2025
-
How To Calculate Current In A Series Circuit
Apr 01, 2025
-
Unity Of Life And Diversity Of Life
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Why Does Km Increase In Competitive Inhibition . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
