4 Most Common Elements In Living Organisms
Muz Play
Mar 23, 2025 · 6 min read
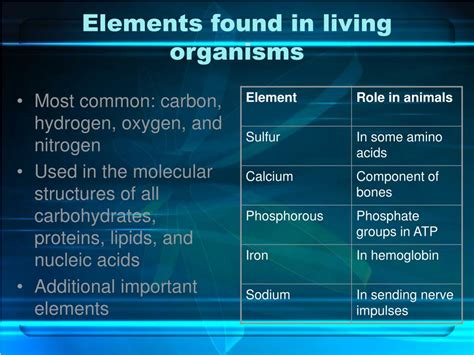
Table of Contents
- 4 Most Common Elements In Living Organisms
- Table of Contents
- 4 Most Common Elements in Living Organisms: A Deep Dive into Carbon, Hydrogen, Oxygen, and Nitrogen
- Carbon: The Backbone of Life
- Carbon's Versatility: From Simple to Complex
- Carbon's Role in the Carbon Cycle
- Hydrogen: The Universal Solvent's Partner
- Hydrogen's Importance in Water and Organic Molecules
- Oxygen: The Breath of Life
- Oxygen's Role in Cellular Respiration and Antioxidant Defense
- Nitrogen: The Building Block of Proteins and Nucleic Acids
- Nitrogen's Role in the Nitrogen Cycle
- The Interplay of the Four Elements
- Conclusion: The Foundation of Life
- Latest Posts
- Latest Posts
- Related Post
4 Most Common Elements in Living Organisms: A Deep Dive into Carbon, Hydrogen, Oxygen, and Nitrogen
Life on Earth is incredibly diverse, ranging from microscopic bacteria to towering redwood trees and complex human beings. Despite this vast variety, all living organisms share a fundamental similarity: their composition. While thousands of different elements exist in the universe, only a handful are crucial for building and maintaining life as we know it. This article will explore the four most common elements found in living organisms: carbon, hydrogen, oxygen, and nitrogen, delving into their individual roles and the synergistic interplay that makes life possible.
Carbon: The Backbone of Life
Carbon is arguably the most important element for life. Its unique properties make it the ideal building block for the vast array of complex molecules that form the basis of all living organisms. The reason for carbon's dominance lies in its ability to form four strong covalent bonds. This tetravalency allows carbon atoms to bond with other carbon atoms, forming long chains and branching structures—a characteristic feature of organic molecules.
Carbon's Versatility: From Simple to Complex
This versatility is unparalleled. Carbon can form single, double, and triple bonds, leading to a wide range of molecular shapes and functionalities. These diverse structures are crucial for creating the complex molecules necessary for life, including:
-
Carbohydrates: These are essential energy sources and structural components in living organisms. Simple sugars like glucose are made up of carbon, hydrogen, and oxygen atoms arranged in specific configurations. Complex carbohydrates, like starch and cellulose, are polymers of simpler sugar units.
-
Lipids (Fats and Oils): Lipids are crucial for energy storage, cell membrane formation, and hormone production. They consist primarily of carbon, hydrogen, and oxygen, although the proportion of oxygen is significantly lower than in carbohydrates. The long hydrocarbon chains in lipids contribute to their hydrophobic nature, a crucial property for membrane function.
-
Proteins: Proteins are the workhorses of the cell, responsible for a vast array of functions, including catalysis (enzymes), structural support, transport, and cell signaling. Proteins are polymers of amino acids, each containing a central carbon atom bonded to an amino group, a carboxyl group, a hydrogen atom, and a variable side chain (R group). The diversity of R groups allows for the vast array of protein structures and functions.
-
Nucleic Acids (DNA and RNA): These molecules carry the genetic information of all living organisms. DNA and RNA are composed of nucleotides, each containing a sugar (deoxyribose in DNA, ribose in RNA), a phosphate group, and a nitrogenous base (adenine, guanine, cytosine, thymine, or uracil). The sequence of nucleotides determines the genetic code.
Carbon's Role in the Carbon Cycle
Carbon is not static; it cycles continuously through the environment. The carbon cycle involves the exchange of carbon between the atmosphere, oceans, land, and living organisms. Photosynthesis, a crucial process performed by plants and some bacteria, incorporates atmospheric carbon dioxide into organic molecules, while respiration releases carbon dioxide back into the atmosphere. The carbon cycle is vital for maintaining the balance of carbon in the biosphere and regulating Earth's climate.
Hydrogen: The Universal Solvent's Partner
Hydrogen, the most abundant element in the universe, is also a crucial component of life. It is significantly lighter than other elements, and its single electron allows it to form a single covalent bond. While not as structurally versatile as carbon, hydrogen's prevalence and its role in forming polar bonds are essential.
Hydrogen's Importance in Water and Organic Molecules
Hydrogen's most crucial role is in the formation of water (H₂O). Water is the universal solvent, facilitating numerous biochemical reactions within cells. Its polar nature, due to the electronegativity difference between oxygen and hydrogen, allows it to dissolve many ionic and polar compounds, making it the ideal medium for biological processes. Furthermore, water's high specific heat capacity helps regulate temperature fluctuations in living organisms.
Beyond water, hydrogen is a key component of all organic molecules. It’s found in carbohydrates, lipids, proteins, and nucleic acids, contributing to the overall structure and functionality of these essential biomolecules. The presence of hydrogen in hydrocarbons, for example, influences their solubility and reactivity.
Oxygen: The Breath of Life
Oxygen is the third most abundant element in living organisms and plays a vital role in cellular respiration, the process that releases energy from organic molecules. Oxygen is highly electronegative, meaning it strongly attracts electrons, leading to the formation of strong polar covalent bonds.
Oxygen's Role in Cellular Respiration and Antioxidant Defense
Cellular respiration is an essential process for energy production in most living organisms. Oxygen acts as the final electron acceptor in the electron transport chain, a series of redox reactions that generate ATP (adenosine triphosphate), the primary energy currency of the cell. Without oxygen, cellular respiration would be significantly less efficient, limiting the energy available to power life's processes.
Moreover, oxygen plays a critical role in antioxidant defense systems. Reactive oxygen species (ROS), such as superoxide radicals and hydrogen peroxide, are byproducts of cellular metabolism that can damage cellular components. Antioxidants, including enzymes like superoxide dismutase and catalase, work to neutralize these ROS, protecting cells from oxidative stress.
Nitrogen: The Building Block of Proteins and Nucleic Acids
Nitrogen is a crucial component of proteins and nucleic acids, making it essential for building and maintaining the genetic material and cellular machinery of life. Nitrogen is found in the amino group of amino acids and in the nitrogenous bases of nucleotides. Its incorporation into these molecules is critical for the structure and function of proteins and nucleic acids.
Nitrogen's Role in the Nitrogen Cycle
Like carbon, nitrogen also undergoes a crucial biogeochemical cycle: the nitrogen cycle. This cycle involves the transformation of nitrogen between different forms, including atmospheric nitrogen (N₂), ammonia (NH₃), nitrates (NO₃⁻), and nitrites (NO₂⁻). Nitrogen fixation, carried out by certain bacteria, converts atmospheric nitrogen into ammonia, making it available to plants. Plants then use ammonia to synthesize amino acids and other nitrogen-containing compounds, which are then passed on to animals through the food chain. Decomposition of organic matter releases nitrogen back into the environment, completing the cycle. Nitrogen limitation often restricts plant growth and overall ecosystem productivity.
The Interplay of the Four Elements
These four elements – carbon, hydrogen, oxygen, and nitrogen – don't function in isolation. They work synergistically to create the complex molecules that underlie life’s processes. The specific ratios of these elements vary between different biomolecules, reflecting the diverse functionalities of these molecules. For example, carbohydrates have a higher proportion of oxygen compared to lipids, reflecting their roles as energy sources versus energy storage. The intricate interplay of these four elements highlights the remarkable efficiency and precision of biological systems.
Conclusion: The Foundation of Life
The four most common elements in living organisms – carbon, hydrogen, oxygen, and nitrogen – form the very foundation of life. Their unique chemical properties, combined with their intricate interactions, allow for the formation of a vast array of complex molecules, enabling the diversity and complexity of life on Earth. Understanding the roles of these elements and their cycling within ecosystems is crucial for appreciating the delicate balance of life and for addressing global challenges like climate change and resource management. Further research into the interplay of these elements promises to unveil even more secrets of life's intricate mechanisms and help us better understand and protect our planet.
Latest Posts
Latest Posts
-
Changing The Order Of Integration Triple Integrals
Mar 26, 2025
-
What Is The Principle Of Cross Cutting Relationships
Mar 26, 2025
-
Limits At Infinity And Infinite Limits
Mar 26, 2025
-
A Process With A Calculated Positive Q
Mar 26, 2025
-
Writing Geometric Series In Summation Notation
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about 4 Most Common Elements In Living Organisms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
