A Bond Where Electrons Are Shared Equally
Muz Play
Mar 17, 2025 · 6 min read
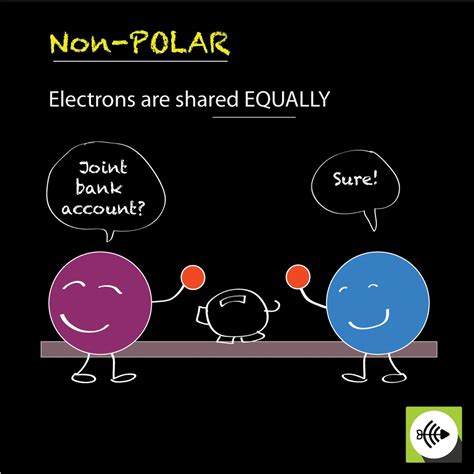
Table of Contents
- A Bond Where Electrons Are Shared Equally
- Table of Contents
- A Bond Where Electrons Are Shared Equally: Understanding Nonpolar Covalent Bonds
- What is a Nonpolar Covalent Bond?
- The Role of Electronegativity
- Distinguishing Nonpolar Covalent Bonds from Polar Covalent and Ionic Bonds
- Characteristics of Nonpolar Covalent Bonds
- Examples of Nonpolar Covalent Bonds
- Significance of Nonpolar Covalent Bonds
- Advanced Concepts: Bond Length and Bond Energy in Nonpolar Covalent Bonds
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
A Bond Where Electrons Are Shared Equally: Understanding Nonpolar Covalent Bonds
A fundamental concept in chemistry is the chemical bond, the force that holds atoms together to form molecules and compounds. Among the various types of chemical bonds, the nonpolar covalent bond stands out as a crucial example of electron sharing. This article delves deep into the intricacies of nonpolar covalent bonds, exploring their formation, properties, examples, and significance in the world around us. We will unravel the nuances of electronegativity, its role in determining bond polarity, and how it distinguishes nonpolar covalent bonds from their polar counterparts.
What is a Nonpolar Covalent Bond?
A nonpolar covalent bond is a type of chemical bond where two atoms share electrons equally. This equal sharing ensures that there is no significant difference in the electronegativity of the atoms involved. Electronegativity, simply put, is the measure of an atom's ability to attract electrons towards itself within a chemical bond. When two atoms have similar electronegativities, they pull on the shared electrons with approximately the same force, resulting in a balanced distribution of charge.
The Role of Electronegativity
Understanding electronegativity is paramount to grasping the concept of nonpolar covalent bonds. Elements on the periodic table exhibit varying electronegativities. Generally, electronegativity increases across a period (from left to right) and decreases down a group (from top to bottom). This trend is a consequence of the effective nuclear charge and atomic radius. Elements with high electronegativity, such as fluorine, oxygen, and nitrogen, strongly attract electrons. Conversely, elements with low electronegativity, such as alkali metals and alkaline earth metals, have a weaker pull on electrons.
When two atoms with similar electronegativities bond, they share the electrons relatively equally, forming a nonpolar covalent bond. The difference in electronegativity (ΔEN) between the two atoms is ideally zero, or very close to zero, typically less than 0.5. This near-equal sharing of electrons prevents the formation of significant partial charges on the atoms, resulting in a nonpolar molecule.
Distinguishing Nonpolar Covalent Bonds from Polar Covalent and Ionic Bonds
It's crucial to differentiate nonpolar covalent bonds from other types of chemical bonds:
-
Polar Covalent Bonds: In contrast to nonpolar covalent bonds, polar covalent bonds involve an unequal sharing of electrons. This unequal sharing occurs when there's a significant difference in electronegativity between the two atoms. The atom with higher electronegativity attracts the shared electrons more strongly, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the other atom. Water (H₂O) is a classic example of a molecule with polar covalent bonds.
-
Ionic Bonds: Ionic bonds are formed when one atom completely transfers one or more electrons to another atom. This transfer creates ions: a positively charged cation and a negatively charged anion. The electrostatic attraction between these oppositely charged ions forms the ionic bond. Table salt (NaCl) is a typical example of an ionic compound.
Characteristics of Nonpolar Covalent Bonds
Nonpolar covalent bonds possess several distinct characteristics:
-
Equal Electron Sharing: As previously emphasized, the defining feature is the equal sharing of electrons between the two atoms.
-
No Dipole Moment: Due to the symmetrical distribution of charge, nonpolar molecules have a zero dipole moment. A dipole moment is a measure of the separation of positive and negative charges within a molecule.
-
Low Melting and Boiling Points: Generally, substances held together by nonpolar covalent bonds have relatively low melting and boiling points compared to ionic or polar covalent compounds. This is because the intermolecular forces (forces between molecules) are weak in nonpolar substances.
-
Poor Solubility in Water: Nonpolar covalent compounds tend to be insoluble or only slightly soluble in water, a polar solvent. This is because "like dissolves like" – polar solvents dissolve polar solutes, and nonpolar solvents dissolve nonpolar solutes.
-
Good Solubility in Nonpolar Solvents: Conversely, nonpolar covalent compounds readily dissolve in nonpolar solvents such as hexane or benzene.
Examples of Nonpolar Covalent Bonds
Many molecules exhibit nonpolar covalent bonds. Here are some notable examples:
-
Diatomic molecules: The most straightforward examples are diatomic molecules composed of two atoms of the same element, such as:
- Hydrogen (H₂): Two hydrogen atoms share their single electrons equally.
- Oxygen (O₂): Oxygen atoms share electrons to form a double bond, but the sharing remains relatively equal.
- Nitrogen (N₂): Nitrogen atoms share electrons to form a triple bond, yet the electronegativity difference is minimal, resulting in a nonpolar bond.
- Chlorine (Cl₂): Similar to the others, chlorine atoms share electrons equally in a single bond.
-
Other molecules: Certain molecules with multiple atoms can also have predominantly nonpolar covalent bonds, provided the atoms have similar electronegativities and the molecule’s geometry leads to a balanced charge distribution. Examples include:
- Methane (CH₄): While carbon and hydrogen have a slight electronegativity difference, it is small enough that methane is considered a nonpolar molecule. The symmetrical tetrahedral geometry further contributes to the balanced charge distribution.
- Carbon dioxide (CO₂): The linear structure and symmetrical distribution of the oxygen atoms around the carbon atom result in a net dipole moment of zero.
- Benzene (C₆H₆): This aromatic hydrocarbon exhibits a very symmetrical structure leading to nonpolar behavior.
Significance of Nonpolar Covalent Bonds
Nonpolar covalent bonds are ubiquitous in nature and play critical roles in various biological and industrial processes:
-
Organic Chemistry: Nonpolar covalent bonds are the backbone of organic chemistry, forming the basis of countless organic molecules, including hydrocarbons, fats, oils, and many polymers. These molecules are essential for life and form the structural components of living organisms.
-
Industrial Applications: Many industrial materials are based on nonpolar molecules. Plastics, for instance, are often composed of long chains of nonpolar carbon-carbon bonds. Nonpolar solvents are extensively used in industrial processes for cleaning, extraction, and dissolving nonpolar substances.
Advanced Concepts: Bond Length and Bond Energy in Nonpolar Covalent Bonds
-
Bond Length: The bond length in a nonpolar covalent bond is determined by the balance between the attractive forces between the nuclei and the shared electrons and the repulsive forces between the two nuclei. Shorter bond lengths typically indicate stronger bonds.
-
Bond Energy: Bond energy represents the amount of energy required to break a covalent bond. In nonpolar covalent bonds, where the electrons are shared equally, the bond energy is generally higher compared to weaker polar covalent bonds, reflecting the stronger attraction between the atoms.
Conclusion
Nonpolar covalent bonds are a cornerstone of chemistry. Their formation and properties are governed by the principle of equal electron sharing between atoms with similar electronegativities. Understanding these bonds is essential for comprehending the behavior of a vast array of substances, from simple diatomic molecules to complex organic compounds and industrial materials. Their significance in the natural world and technological applications underscores their importance in both basic and applied science. The concept of electronegativity serves as the key to differentiating between nonpolar covalent bonds and other types of chemical bonds, allowing us to accurately predict and understand the properties of diverse chemical species. Further exploration into the world of chemical bonding reveals even greater complexity and beauty in the molecular structure of our universe.
Latest Posts
Latest Posts
-
Proof Of One To One Function
Mar 17, 2025
-
What Is The Plasma Membrane Of A Muscle Fiber Called
Mar 17, 2025
-
How To Find The Equivalence Point Of A Titration
Mar 17, 2025
-
What Are The Function Of Family
Mar 17, 2025
-
What Are Characteristics Of A Plant
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about A Bond Where Electrons Are Shared Equally . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
