A Compound Held Together By Ionic Bonds Is Called
Muz Play
Mar 24, 2025 · 6 min read
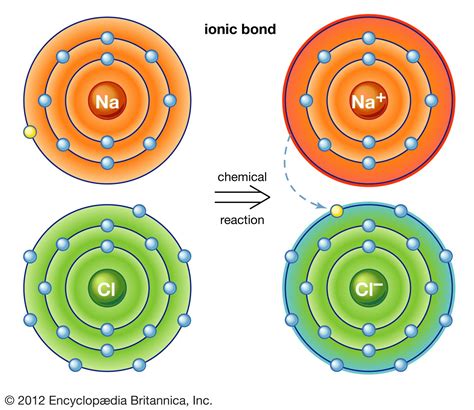
Table of Contents
A Compound Held Together by Ionic Bonds is Called an Ionic Compound
An ionic compound is a chemical compound in which ions are held together in a lattice structure by electrostatic forces termed ionic bonds. These bonds form through the electrostatic attraction between oppositely charged ions, typically a positively charged cation and a negatively charged anion. Understanding ionic compounds requires delving into the fundamental principles of chemical bonding, electron transfer, and crystal structures. This comprehensive guide will explore these aspects in detail, providing a thorough understanding of what makes an ionic compound, its properties, and its significance in various fields.
The Formation of Ionic Bonds: A Transfer of Electrons
The formation of an ionic bond is a fundamental process in chemistry, driven by the tendency of atoms to achieve a stable electron configuration, often resembling that of a noble gas. This stable configuration, typically involving a full outermost electron shell (octet rule), is energetically favorable. Atoms achieve this stability through the gain or loss of electrons.
Electron Transfer: Ionic bonding occurs when one atom, typically a metal, readily donates electrons, becoming a positively charged cation. Simultaneously, another atom, usually a non-metal, readily accepts these electrons, becoming a negatively charged anion. This transfer of electrons is the defining characteristic of ionic bonding.
Electrostatic Attraction: The oppositely charged ions, the cation and the anion, are then attracted to each other through strong electrostatic forces. This attraction is what constitutes the ionic bond. The strength of the ionic bond is directly related to the magnitude of the charges on the ions and the distance between them (Coulomb's Law). Greater charges and shorter distances lead to stronger bonds.
Examples of Ionic Bond Formation
Let's consider the classic example of sodium chloride (NaCl), commonly known as table salt. Sodium (Na), an alkali metal, has one electron in its outermost shell. It readily loses this electron to achieve a stable electron configuration like neon (Ne). Chlorine (Cl), a halogen, has seven electrons in its outermost shell and readily gains one electron to achieve a stable configuration like argon (Ar).
The sodium atom loses an electron to become a sodium cation (Na⁺), and the chlorine atom gains that electron to become a chloride anion (Cl⁻). The electrostatic attraction between the positively charged Na⁺ ion and the negatively charged Cl⁻ ion forms the ionic bond, resulting in the formation of sodium chloride (NaCl).
Properties of Ionic Compounds: A Consequence of Ionic Bonding
The properties of ionic compounds are a direct consequence of the strong electrostatic forces holding the ions together in a crystal lattice. These properties distinguish them from other types of compounds, such as covalent compounds.
High Melting and Boiling Points:
Ionic compounds generally have high melting and boiling points. This is because the strong electrostatic forces between the ions require a significant amount of energy to overcome, thus necessitating high temperatures to break the ionic bonds and transition from solid to liquid or liquid to gas.
Crystalline Structure:
Ionic compounds exist as crystalline solids at room temperature. The ions are arranged in a highly ordered three-dimensional lattice structure, maximizing the electrostatic attraction between oppositely charged ions and minimizing repulsion between like-charged ions. This structure gives ionic compounds their characteristic crystalline shape and often brittle nature.
Hardness and Brittleness:
Ionic compounds are generally hard but brittle. Their hardness stems from the strong ionic bonds holding the crystal lattice together. However, their brittleness is due to the rigid structure. When subjected to stress, the lattice can easily fracture because shifting the ions causes repulsion between like-charged ions, leading to the crystal breaking.
Solubility:
The solubility of ionic compounds varies depending on the specific compound and the solvent. Many ionic compounds are soluble in polar solvents, such as water. This is because the polar water molecules can interact with the charged ions, effectively surrounding and separating them, thus dissolving the ionic compound.
Conductivity:
Ionic compounds are generally poor conductors of electricity in the solid state. This is because the ions are held rigidly in the crystal lattice and cannot move freely to carry an electric current. However, they become good conductors when molten (liquid) or dissolved in a solution. In these states, the ions are mobile and can carry an electric current.
Examples of Common Ionic Compounds
Numerous compounds found in everyday life are ionic compounds. Some notable examples include:
- Sodium chloride (NaCl): Table salt, essential in our diet and used extensively in various industries.
- Calcium carbonate (CaCO₃): A major component of limestone, marble, and shells. It's also used in various industrial applications.
- Potassium chloride (KCl): Used as a salt substitute and in fertilizers.
- Magnesium oxide (MgO): Used in various applications including refractories, medicine, and agriculture.
- Sodium hydroxide (NaOH): A strong base used in numerous industrial processes.
- Ammonium chloride (NH₄Cl): Used in fertilizers, batteries, and as a cleaning agent.
Naming Ionic Compounds: A Systematic Approach
Naming ionic compounds follows a systematic procedure. The name of the cation is stated first, followed by the name of the anion.
-
For cations: The name of the cation is simply the name of the metal. For example, Na⁺ is sodium, and Ca²⁺ is calcium. If the metal can form more than one type of cation (e.g., iron, Fe²⁺ and Fe³⁺), the charge of the cation is indicated using Roman numerals in parentheses after the metal name. For example, Fe²⁺ is iron(II), and Fe³⁺ is iron(III).
-
For anions: The names of monatomic anions (anions formed from a single atom) are formed by adding the suffix "-ide" to the root name of the non-metal. For example, Cl⁻ is chloride, O²⁻ is oxide, and S²⁻ is sulfide. The names of polyatomic anions (anions formed from multiple atoms) follow specific naming conventions, often ending in "-ite" or "-ate" based on the number of oxygen atoms present.
Ionic Compounds in Everyday Life and Industry
Ionic compounds play a crucial role in various aspects of everyday life and industrial processes. Their unique properties make them essential components in diverse applications.
- Food and Nutrition: Sodium chloride (NaCl) is essential in our diet for maintaining electrolyte balance.
- Medicine: Many medications contain ionic compounds that play specific roles in the body. For example, calcium supplements provide calcium ions necessary for bone health.
- Agriculture: Ionic compounds are crucial components in fertilizers, providing essential nutrients for plant growth.
- Industry: Ionic compounds are essential in manufacturing processes, construction materials, and various chemical reactions. For example, sodium hydroxide is used in the production of soap and paper.
- Environmental Science: The study of ionic compounds is vital in understanding environmental processes, such as water quality and soil chemistry.
Conclusion: The Significance of Ionic Compounds
Ionic compounds, held together by the powerful electrostatic forces of ionic bonds, represent a fundamental class of chemical compounds. Their unique properties, arising from the electrostatic interactions between cations and anions, lead to a wide range of applications in diverse fields, from everyday life to advanced technologies. Understanding the formation, properties, and applications of ionic compounds is essential for comprehending various chemical and physical phenomena. The consistent application of systematic naming conventions ensures clear communication and accurate identification of these crucial compounds across scientific disciplines. The ongoing research and development in materials science and chemistry continuously unveil new applications and further elucidate the multifaceted nature of ionic compounds, highlighting their continued importance in scientific endeavors and technological advancements.
Latest Posts
Latest Posts
-
Tipos De Triangulos Segun Sus Angulos
Mar 25, 2025
-
Did The Swahili Coast Require Monsoons To Access
Mar 25, 2025
-
An Irreversible Inhibitor Is One That
Mar 25, 2025
-
When Elements Combine To Form Compounds
Mar 25, 2025
-
Describe The Sampling Distribution Of P Hat
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about A Compound Held Together By Ionic Bonds Is Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
