An Irreversible Inhibitor Is One That
Muz Play
Mar 25, 2025 · 5 min read
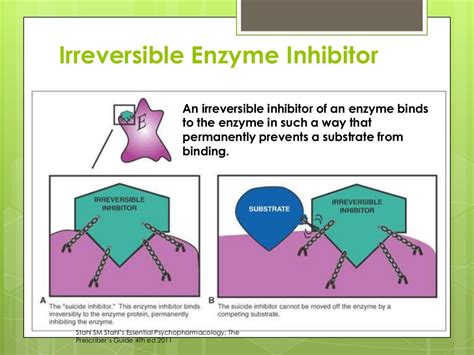
Table of Contents
Irreversible Inhibitors: A Deep Dive into Enzyme Inactivation
Irreversible inhibitors, unlike their reversible counterparts, form a stable, covalent bond with their target enzyme, permanently inactivating it. This permanent inactivation distinguishes them from reversible inhibitors, which can dissociate from the enzyme, allowing the enzyme to regain its activity. Understanding the mechanisms and implications of irreversible inhibition is crucial in various fields, including drug discovery, biochemistry, and toxicology. This article delves deep into the nature of irreversible inhibitors, exploring their mechanisms of action, applications, and significance.
Mechanisms of Irreversible Inhibition
Irreversible inhibitors achieve their effect through several distinct mechanisms, all leading to the permanent inactivation of the enzyme. These mechanisms often involve the formation of a strong covalent bond between the inhibitor and the enzyme.
1. Covalent Modification of Active Site Residues
Many irreversible inhibitors work by covalently modifying crucial amino acid residues within the enzyme's active site. This modification can alter the active site's shape and charge, preventing substrate binding and catalysis. Common reactive groups in irreversible inhibitors that participate in covalent bond formation include:
-
Alkyl halides: These compounds can alkylate nucleophilic residues like cysteine, serine, or histidine. The alkylation reaction permanently modifies the residue, disrupting the enzyme's function. Iodoacetamide is a classic example of an alkylating agent used to study cysteine residues in enzymes.
-
Epoxides: These three-membered ring structures are highly reactive and can open to form covalent bonds with nucleophilic residues in the active site.
-
Phosphate esters: Organophosphates, such as those found in certain insecticides and nerve agents, are potent inhibitors that phosphorylate serine residues in the active site of acetylcholinesterase, a crucial enzyme in the nervous system. This irreversible inhibition leads to the accumulation of acetylcholine, resulting in severe neurological effects.
-
Michael acceptors: These compounds contain an electrophilic carbon atom that can react with nucleophilic residues such as cysteine or lysine. Acrylamide is a commonly used example, although it's important to note that it is toxic.
2. Transition State Analogs
While many irreversible inhibitors utilize covalent modification, some mimic the transition state of the enzyme-catalyzed reaction. These transition state analogs bind very tightly to the enzyme's active site, often forming a stable, irreversible complex. The prolonged occupancy of the active site effectively prevents the enzyme from binding its natural substrate. This strategy exploits the enzyme's high affinity for the transition state. While not strictly covalent, the extremely tight binding leads to functional irreversibility.
3. Mechanism-Based Inhibitors (Suicide Inhibitors)
Mechanism-based inhibitors, also known as suicide inhibitors, represent a particularly clever class of irreversible inhibitors. These inhibitors are initially recognized and bound by the enzyme as if they were normal substrates. However, during the normal course of the enzymatic reaction, the inhibitor undergoes a chemical transformation within the active site, forming a highly reactive intermediate that then covalently modifies the enzyme. This "self-destruction" of the inhibitor leads to its irreversible binding to the enzyme.
The effectiveness of mechanism-based inhibitors lies in their ability to exploit the enzyme's own catalytic machinery to achieve irreversible inactivation. This selective targeting mechanism makes them particularly attractive for drug development.
Classes of Irreversible Inhibitors and their Applications
Irreversible inhibitors have found wide-ranging applications in various fields. Their specific applications often depend on the target enzyme and the desired outcome.
1. Inhibitors of Serine Proteases
Serine proteases, a large family of enzymes with a crucial serine residue in their active site, are targeted by many irreversible inhibitors. These enzymes are involved in diverse biological processes, and their inhibition has implications in treating various diseases.
-
Organophosphates: As mentioned earlier, these are potent inhibitors of acetylcholinesterase, a serine protease, and are used (though with caution due to toxicity) as insecticides and nerve agents. The irreversible inhibition leads to the accumulation of acetylcholine, causing paralysis and death.
-
Protease inhibitors in HIV treatment: Many antiretroviral drugs used to treat HIV/AIDS are irreversible inhibitors of viral proteases. These viral proteases are essential for the virus's replication cycle, and their inhibition effectively prevents viral replication. Examples include saquinavir and ritonavir.
2. Inhibitors of Other Enzyme Classes
Irreversible inhibitors are not limited to serine proteases; they can target other enzyme classes as well:
-
Aspartic proteases: Inhibitors of aspartic proteases are used in the treatment of certain viral infections.
-
Matrix metalloproteinases (MMPs): MMPs are involved in tissue degradation, and their inhibition is being explored for the treatment of diseases like cancer and arthritis. However, the development of specific and safe inhibitors for this class of enzymes presents challenges.
-
Topoisomerases: These enzymes are involved in DNA replication and repair, and their inhibition by certain drugs, such as camptothecin, is used in cancer chemotherapy. However, the inhibition of topoisomerases can also have side effects due to their essential role in cellular processes.
Implications and Considerations
The use of irreversible inhibitors has several crucial implications that must be considered.
-
Toxicity: Many irreversible inhibitors, particularly those used as pesticides or nerve agents, are highly toxic. Their irreversible nature means that the affected enzyme cannot recover its activity.
-
Specificity: While desirable, achieving high specificity is challenging. Off-target effects can lead to adverse reactions. Careful design and testing are crucial to minimize non-specific inhibition.
-
Reversibility (or lack thereof): The irreversibility of the inhibition poses challenges in controlling the extent and duration of the effect. Once the inhibitor has bound, the effect is essentially permanent, leading to potential long-term consequences.
Conclusion
Irreversible inhibitors play a pivotal role in various fields, from drug development to biochemical research. Their ability to permanently inactivate enzymes makes them potent tools, but careful consideration of their toxicity and specificity is essential. The diverse mechanisms of action, coupled with ongoing research into new and improved inhibitors, continue to shape our understanding of enzyme function and open new avenues for therapeutic intervention and scientific advancement. The development of selective and safe irreversible inhibitors remains a critical area of research, promising innovative solutions for a wide range of diseases and technological applications. Further research focusing on improving the specificity and reducing the toxicity of irreversible inhibitors will undoubtedly contribute to their wider and safer use in various applications.
Latest Posts
Latest Posts
-
An Introduction To General Organic And Biological Chemistry
Mar 26, 2025
-
The Cutaneous Membrane Is Also Known As
Mar 26, 2025
-
Solve The Following System Of Equations Algebraically
Mar 26, 2025
-
Why Are Tertiary Carbocations More Stable
Mar 26, 2025
-
How To Draw An Integral Sign
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about An Irreversible Inhibitor Is One That . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
