A Hydrocarbon Is Any Compound That Contains
Muz Play
Mar 28, 2025 · 7 min read
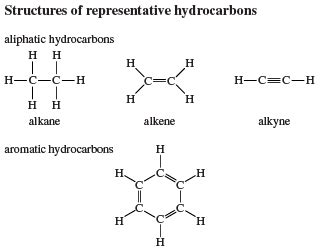
Table of Contents
A Hydrocarbon is Any Compound That Contains: Exploring the World of Organic Chemistry
Hydrocarbons form the bedrock of organic chemistry, representing a vast and diverse class of compounds. Understanding what defines a hydrocarbon, their properties, types, and applications is crucial for anyone venturing into the fascinating world of organic molecules. This comprehensive guide delves deep into the subject, providing a detailed overview for both beginners and those seeking a refresher.
What Defines a Hydrocarbon?
At its core, a hydrocarbon is simply any compound that contains only two elements: carbon (C) and hydrogen (H). These elements bond together covalently, forming chains, branches, and rings of varying complexities. The sheer number of possible arrangements of these two atoms leads to the immense variety observed within the hydrocarbon family. This diversity is reflected in the wide range of physical and chemical properties exhibited by hydrocarbons, shaping their diverse applications in various industries.
The Backbone of Organic Molecules: Carbon's Unique Role
Carbon's exceptional ability to form four covalent bonds is the key to the vastness of hydrocarbon chemistry. This tetravalency allows carbon atoms to link together in long chains, branched structures, and cyclic rings, creating the fundamental frameworks for countless organic molecules. The carbon-carbon bonds can be single, double, or triple bonds, influencing the molecule's shape and reactivity. Hydrogen atoms then fill the remaining bonding sites on the carbon atoms, completing the hydrocarbon structure.
Types of Hydrocarbons: A Detailed Breakdown
Hydrocarbons are broadly classified into two main categories based on their bonding structure:
1. Aliphatic Hydrocarbons: Chains and Branches
Aliphatic hydrocarbons comprise open-chain or branched structures and are further subdivided into:
-
Alkanes: These are the simplest hydrocarbons, containing only single carbon-carbon bonds. They are also known as saturated hydrocarbons because each carbon atom is bonded to the maximum number of hydrogen atoms. The general formula for alkanes is C<sub>n</sub>H<sub>2n+2</sub>, where 'n' represents the number of carbon atoms. Examples include methane (CH<sub>4</sub>), ethane (C<sub>2</sub>H<sub>6</sub>), propane (C<sub>3</sub>H<sub>8</sub>), and butane (C<sub>4</sub>H<sub>10</sub>). Alkanes are relatively unreactive, primarily undergoing combustion reactions.
-
Alkenes: Alkenes contain at least one carbon-carbon double bond (C=C). The presence of this double bond introduces unsaturation into the molecule, making alkenes more reactive than alkanes. The general formula for alkenes is C<sub>n</sub>H<sub>2n</sub>. Ethylene (C<sub>2</sub>H<sub>4</sub>), the simplest alkene, is a crucial building block in the petrochemical industry. Alkenes readily undergo addition reactions, where atoms or groups are added across the double bond.
-
Alkynes: Alkynes are characterized by the presence of at least one carbon-carbon triple bond (C≡C). They are even more unsaturated than alkenes and exhibit higher reactivity. The general formula for alkynes is C<sub>n</sub>H<sub>2n-2</sub>. Acetylene (C<sub>2</sub>H<sub>2</sub>) is the simplest alkyne and finds applications in welding and cutting due to its high heat of combustion. Similar to alkenes, alkynes undergo addition reactions.
2. Aromatic Hydrocarbons: The Ring of Stability
Aromatic hydrocarbons, also known as arenes, are characterized by the presence of a benzene ring or related structures. Benzene (C<sub>6</sub>H<sub>6</sub>) is the simplest aromatic hydrocarbon, featuring a six-membered ring with alternating single and double bonds. This arrangement, known as resonance, leads to exceptional stability and unique chemical properties. Aromatic hydrocarbons are less reactive than alkenes and alkynes but can undergo substitution reactions, where a hydrogen atom is replaced by another atom or group. Examples of aromatic hydrocarbons include toluene, naphthalene, and anthracene.
Isomerism in Hydrocarbons: The Same Formula, Different Structures
Isomerism is a prevalent phenomenon in hydrocarbon chemistry. Isomers are molecules with the same molecular formula but different structural arrangements. This structural variation leads to differences in physical and chemical properties. There are several types of isomerism in hydrocarbons, including:
-
Chain isomerism: This type of isomerism arises from variations in the carbon chain's structure, such as straight-chain versus branched-chain arrangements. For example, butane (C<sub>4</sub>H<sub>10</sub>) exists as both a straight-chain isomer (n-butane) and a branched-chain isomer (isobutane).
-
Positional isomerism: This occurs when the position of a functional group (e.g., a double bond or a triple bond) varies within the same carbon skeleton.
-
Functional group isomerism: This refers to isomers with the same molecular formula but different functional groups. For example, an alcohol and an ether can be functional group isomers.
-
Stereoisomerism: This involves isomers with the same connectivity but different spatial arrangements of atoms. Geometric isomerism (cis-trans isomerism) and optical isomerism are common types of stereoisomerism found in hydrocarbons with double bonds or chiral centers.
Properties of Hydrocarbons: A Range of Characteristics
The properties of hydrocarbons vary significantly depending on their structure, specifically the length of the carbon chain and the presence of unsaturation. Some key properties include:
-
Physical state: Smaller hydrocarbons (e.g., methane, ethane, propane) are gases at room temperature. Medium-sized hydrocarbons are liquids, while larger hydrocarbons are solids. The boiling points and melting points generally increase with increasing chain length.
-
Solubility: Hydrocarbons are nonpolar molecules and are therefore insoluble in water (a polar solvent). They are, however, soluble in nonpolar solvents like other hydrocarbons and organic solvents.
-
Density: Hydrocarbons are generally less dense than water, meaning they float on water.
-
Combustion: Hydrocarbons are readily combustible, reacting with oxygen to produce carbon dioxide, water, and a large amount of heat. This property is exploited in various applications, from heating to powering vehicles.
-
Reactivity: The reactivity of hydrocarbons depends on their structure. Alkanes are relatively unreactive, while alkenes and alkynes are more reactive due to the presence of double or triple bonds. Aromatic hydrocarbons exhibit intermediate reactivity.
Applications of Hydrocarbons: A Wide Spectrum of Uses
Hydrocarbons play a crucial role in modern society, finding applications in a vast array of industries. Their widespread use stems from their abundance, relatively low cost, and diverse properties. Some notable applications include:
-
Fuels: Hydrocarbons are the primary source of energy for transportation, heating, and electricity generation. Fossil fuels like petroleum and natural gas are rich sources of hydrocarbons.
-
Petrochemicals: The petrochemical industry uses hydrocarbons as raw materials to produce plastics, synthetic fibers, solvents, and countless other products. Ethylene and propylene are particularly important building blocks in this industry.
-
Lubricants: Certain hydrocarbons are used as lubricants in engines and machinery, reducing friction and wear.
-
Solvents: Many hydrocarbons serve as solvents in various applications, dissolving nonpolar substances.
-
Pharmaceuticals and other specialized chemicals: Hydrocarbons serve as starting materials for the synthesis of a wide range of pharmaceuticals, pesticides, and other specialty chemicals.
Environmental Concerns Related to Hydrocarbons: Striking a Balance
While hydrocarbons are essential to modern life, their use raises significant environmental concerns. The combustion of hydrocarbons releases greenhouse gases like carbon dioxide, contributing to climate change. Furthermore, the extraction and processing of fossil fuels can cause environmental damage through habitat destruction, water pollution, and air pollution. Sustainable practices, renewable energy sources, and the development of alternative materials are crucial for mitigating the negative environmental impacts associated with hydrocarbon use.
The Future of Hydrocarbon Research: Innovation and Sustainability
Research in hydrocarbon chemistry continues to advance, focusing on developing more efficient and sustainable methods for their production, utilization, and disposal. Areas of active research include:
-
Developing cleaner combustion technologies: Research focuses on minimizing harmful emissions from hydrocarbon combustion, including greenhouse gases and pollutants.
-
Exploring alternative sources of hydrocarbons: Scientists are investigating alternative sources of hydrocarbons, such as biomass and algae, to reduce reliance on fossil fuels.
-
Designing biodegradable and recyclable hydrocarbon-based materials: This research aims to reduce the environmental impact of plastic and other hydrocarbon-derived materials by developing sustainable alternatives.
-
Developing new catalytic processes: Catalysts play a critical role in improving the efficiency and selectivity of hydrocarbon processing, leading to reduced energy consumption and waste generation.
Conclusion: Hydrocarbons – A Fundamental Cornerstone of Chemistry and Society
In conclusion, hydrocarbons form the basis of a vast and complex branch of chemistry with significant societal impact. Understanding their structure, properties, and applications is essential for addressing various challenges related to energy, environmental sustainability, and material science. Ongoing research promises to unlock new possibilities for leveraging the power of hydrocarbons while minimizing their negative environmental effects, shaping a future where both progress and sustainability coexist.
Latest Posts
Latest Posts
-
What Is A Reference Group In Sociology
Mar 31, 2025
-
Dividing Polynomials Math Lib Answer Key
Mar 31, 2025
-
What Is Held Constant In Gay Lussacs Law
Mar 31, 2025
-
Example Of A Line In A Poem
Mar 31, 2025
-
Interval Of Convergence Of A Taylor Series
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about A Hydrocarbon Is Any Compound That Contains . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
