What Is Held Constant In Gay Lussac's Law
Muz Play
Mar 31, 2025 · 5 min read
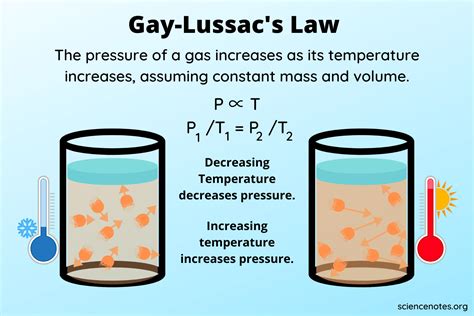
Table of Contents
What is Held Constant in Gay-Lussac's Law? A Deep Dive into Pressure-Temperature Relationships
Gay-Lussac's Law, also known as Amontons' Law, is a fundamental gas law that describes the relationship between the pressure and temperature of a gas when the volume and amount of gas are held constant. Understanding what remains constant is crucial to applying the law correctly and interpreting its implications. This article will delve deep into Gay-Lussac's Law, explaining its core principles, the importance of constant volume and amount of gas, exploring practical applications, and addressing common misconceptions.
Understanding Gay-Lussac's Law: The Pressure-Temperature Connection
Gay-Lussac's Law states that the pressure of a given amount of gas held at constant volume is directly proportional to its absolute temperature. This means that as the temperature of the gas increases, its pressure also increases proportionally, and vice versa. This relationship can be mathematically expressed as:
P₁/T₁ = P₂/T₂
Where:
- P₁ represents the initial pressure of the gas.
- T₁ represents the initial absolute temperature of the gas (in Kelvin).
- P₂ represents the final pressure of the gas.
- T₂ represents the final absolute temperature of the gas (in Kelvin).
This equation is only valid when the volume (V) and the number of moles (n) of the gas remain constant throughout the process. This crucial condition forms the bedrock of the law. Let's explore why.
The Importance of Constant Volume (V)
Maintaining a constant volume is paramount to Gay-Lussac's Law. If the volume changes, the gas particles have more or less space to move, directly affecting the frequency and force of collisions with the container walls. These collisions are what constitute pressure. An increase in volume, even with a constant temperature, would lead to a decrease in pressure as the particles are more spread out. Similarly, a decrease in volume would increase pressure.
Imagine a sealed container filled with gas. If we heat the container, the gas particles gain kinetic energy and move faster. They collide with the container walls more frequently and with greater force, resulting in an increase in pressure. However, if the container were allowed to expand, the increased kinetic energy would be partially absorbed by the increased volume, reducing the overall impact on pressure. Thus, a constant volume ensures that the observed pressure change directly reflects the temperature change.
The Importance of Constant Amount of Gas (n)
Keeping the amount of gas constant is equally crucial. If we add more gas to the container, even at a constant temperature and volume, we increase the number of particles colliding with the walls. This leads to a pressure increase independent of temperature changes. Conversely, removing gas reduces the number of collisions and lowers the pressure.
Therefore, a constant amount of gas ensures that the pressure change is solely attributed to the temperature variation. Any change in the number of gas molecules (moles) would introduce a confounding variable, invalidating the direct proportionality described by Gay-Lussac's Law.
Practical Applications of Gay-Lussac's Law
Gay-Lussac's Law finds numerous practical applications in various fields:
1. Pressure Cookers:
Pressure cookers operate based on Gay-Lussac's Law. The sealed container prevents volume changes. As the temperature increases during cooking, the pressure inside the cooker rises significantly, leading to faster cooking times.
2. Tire Pressure:
On a hot day, the air inside car tires heats up. Since the tire's volume remains relatively constant, the pressure inside increases. This is why tire pressure needs to be checked regularly and adjusted accordingly based on temperature variations.
3. Aerosol Cans:
Aerosol cans are another example. The propellant inside is under pressure. Changes in temperature can significantly affect the pressure inside the can, making it potentially dangerous if exposed to extreme heat.
4. Meteorology:
Meteorologists use Gay-Lussac's Law to understand and predict weather patterns. Temperature changes in the atmosphere directly influence atmospheric pressure, impacting weather systems.
5. Industrial Processes:
Many industrial processes involving gases, such as chemical reactions and gas storage, require precise control of temperature and pressure. Gay-Lussac's Law provides the theoretical framework for managing these parameters effectively.
Common Misconceptions about Gay-Lussac's Law
Several misconceptions often surround Gay-Lussac's Law. It's important to clarify these to ensure a complete understanding:
1. Applicability to all gases:
Gay-Lussac's Law is an ideal gas law and works best for ideal gases. Real gases deviate from ideal behavior, especially at high pressures and low temperatures, because of intermolecular forces and molecular volumes.
2. Ignoring the units of temperature:
Using the incorrect temperature scale (e.g., Celsius instead of Kelvin) will lead to incorrect results. Absolute temperature (Kelvin) must always be used as the zero point on the Kelvin scale represents the absence of molecular motion.
3. Assuming constant conditions when they are not:
It is crucial to ensure that both volume and the amount of gas remain truly constant throughout the experiment or process under consideration. Any deviation from these conditions will invalidate the application of the law.
Conclusion: The Foundation of Understanding Gas Behavior
Gay-Lussac's Law is a cornerstone of our understanding of gas behavior. The law's precise applicability hinges on maintaining constant volume and the amount of gas. By appreciating the significance of these constant factors, we can accurately predict and control gas pressure changes in response to temperature fluctuations. Its practical applications are diverse, impacting our daily lives and various industrial processes. However, it is essential to be aware of the limitations of the law, particularly when dealing with real gases under extreme conditions. A thorough grasp of Gay-Lussac's Law, coupled with an understanding of its underlying assumptions and limitations, allows for a more comprehensive understanding of the world around us.
Latest Posts
Latest Posts
-
How To Calculate Standard Free Energy Change
Apr 01, 2025
-
Root Mean Square Velocity Of Gas
Apr 01, 2025
-
Where Is The Respiratory Center Located In The Brain
Apr 01, 2025
-
What Is The Difference Between Cellular Respiration And Fermentation
Apr 01, 2025
-
What Are Two Functional Groups Found In Amino Acids
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Is Held Constant In Gay Lussac's Law . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
