How To Calculate Standard Free Energy Change
Muz Play
Apr 01, 2025 · 6 min read
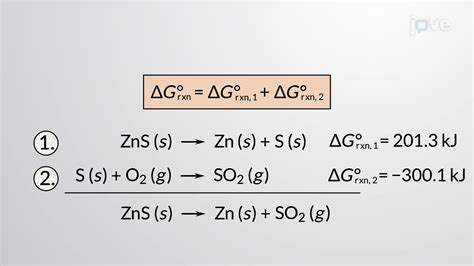
Table of Contents
How to Calculate Standard Free Energy Change: A Comprehensive Guide
Standard free energy change (ΔG°) is a crucial thermodynamic concept that predicts the spontaneity of a reaction under standard conditions (298 K and 1 atm pressure). Understanding how to calculate it is fundamental to various fields, including chemistry, biochemistry, and materials science. This comprehensive guide will walk you through the different methods of calculating ΔG°, exploring the underlying principles and providing practical examples.
Understanding Gibbs Free Energy and Spontaneity
Before diving into the calculations, let's establish a clear understanding of Gibbs Free Energy (G) and its relationship to spontaneity. Gibbs Free Energy represents the maximum amount of reversible work that can be performed by a system at constant temperature and pressure. The change in Gibbs Free Energy (ΔG) determines the spontaneity of a process:
-
ΔG < 0: The reaction is spontaneous (exergonic) under the given conditions. The reaction will proceed in the forward direction without external input.
-
ΔG = 0: The reaction is at equilibrium. There is no net change in the concentrations of reactants and products.
-
ΔG > 0: The reaction is non-spontaneous (endergonic) under the given conditions. The reaction will not proceed in the forward direction without external input (like energy from another reaction or an external energy source).
Methods for Calculating Standard Free Energy Change (ΔG°)
There are several ways to calculate the standard free energy change, depending on the available information. Let's explore the most common methods:
Method 1: Using Standard Free Energy of Formation (ΔG°f)
This is arguably the most straightforward method, particularly for complex reactions. The standard free energy of formation (ΔG°f) is the change in free energy that accompanies the formation of one mole of a substance from its constituent elements in their standard states. The standard free energy change of a reaction can be calculated using the following equation:
ΔG°<sub>rxn</sub> = Σ ΔG°<sub>f</sub>(products) - Σ ΔG°<sub>f</sub>(reactants)
Where:
- ΔG°<sub>rxn</sub> is the standard free energy change of the reaction.
- ΔG°<sub>f</sub>(products) is the sum of the standard free energies of formation of the products, each multiplied by its stoichiometric coefficient.
- ΔG°<sub>f</sub>(reactants) is the sum of the standard free energies of formation of the reactants, each multiplied by its stoichiometric coefficient.
Example:
Consider the reaction: CO(g) + ½O₂(g) → CO₂(g)
Given the following standard free energies of formation:
- ΔG°<sub>f</sub>(CO(g)) = -137.2 kJ/mol
- ΔG°<sub>f</sub>(O₂(g)) = 0 kJ/mol (since O₂ is in its standard state)
- ΔG°<sub>f</sub>(CO₂(g)) = -394.4 kJ/mol
Calculation:
ΔG°<sub>rxn</sub> = [(-394.4 kJ/mol)] - [(-137.2 kJ/mol) + ½(0 kJ/mol)] = -257.2 kJ/mol
This negative value indicates that the reaction is spontaneous under standard conditions.
Method 2: Using Standard Enthalpy Change (ΔH°) and Standard Entropy Change (ΔS°)
This method utilizes the relationship between Gibbs Free Energy, enthalpy, and entropy:
ΔG° = ΔH° - TΔS°
Where:
- ΔG° is the standard free energy change.
- ΔH° is the standard enthalpy change (heat of reaction).
- T is the temperature in Kelvin (usually 298 K for standard conditions).
- ΔS° is the standard entropy change.
To use this method, you need to determine ΔH° and ΔS° for the reaction. These values can be obtained from thermodynamic tables or calculated using standard enthalpies and entropies of formation, similar to the method described above.
Example:
Let's use the same reaction as before: CO(g) + ½O₂(g) → CO₂(g)
Suppose we have:
- ΔH° = -283.0 kJ/mol
- ΔS° = -0.087 kJ/mol·K
- T = 298 K
Calculation:
ΔG° = (-283.0 kJ/mol) - (298 K)(-0.087 kJ/mol·K) = -257.0 kJ/mol
The slight difference in the result compared to Method 1 is due to rounding errors in the thermodynamic data.
Method 3: Using Equilibrium Constant (K<sub>eq</sub>)
For reactions at equilibrium, the standard free energy change is related to the equilibrium constant (K<sub>eq</sub>) through the following equation:
ΔG° = -RTlnK<sub>eq</sub>
Where:
- R is the ideal gas constant (8.314 J/mol·K).
- T is the temperature in Kelvin.
- ln denotes the natural logarithm.
This method is particularly useful when the equilibrium constant is known experimentally. A large K<sub>eq</sub> (K<sub>eq</sub> >> 1) indicates a spontaneous reaction (ΔG° < 0), while a small K<sub>eq</sub> (K<sub>eq</sub> << 1) indicates a non-spontaneous reaction (ΔG° > 0).
Example:
If K<sub>eq</sub> for a reaction at 298 K is 10<sup>5</sup>, then:
ΔG° = -(8.314 J/mol·K)(298 K)ln(10<sup>5</sup>) ≈ -28.5 kJ/mol
This negative value suggests a spontaneous reaction.
Factors Affecting Standard Free Energy Change
Several factors influence the standard free energy change of a reaction:
-
Temperature: As shown in the equation ΔG° = ΔH° - TΔS°, temperature significantly impacts ΔG°. The effect of temperature depends on the signs of ΔH° and ΔS°.
-
Enthalpy Change (ΔH°): Exothermic reactions (ΔH° < 0) generally favor spontaneity, while endothermic reactions (ΔH° > 0) often require external energy input.
-
Entropy Change (ΔS°): Reactions that lead to an increase in disorder (ΔS° > 0) tend to be more spontaneous.
Non-Standard Conditions: Calculating ΔG
The calculations above apply to standard conditions. For non-standard conditions, we use the following equation:
ΔG = ΔG° + RTlnQ
Where:
- ΔG is the free energy change under non-standard conditions.
- ΔG° is the standard free energy change.
- R is the ideal gas constant.
- T is the temperature in Kelvin.
- Q is the reaction quotient, which expresses the relative amounts of products and reactants at any given point in the reaction.
Calculating Q requires the concentrations (or partial pressures for gases) of reactants and products at the specific conditions. This equation allows us to predict the spontaneity of a reaction under various conditions, beyond standard state.
Applications of Standard Free Energy Change Calculations
Calculating ΔG° finds wide applications in diverse fields:
-
Predicting Reaction Spontaneity: As previously mentioned, the sign of ΔG° directly indicates whether a reaction will proceed spontaneously under standard conditions.
-
Biochemistry: ΔG° plays a critical role in understanding metabolic pathways and the energetics of biochemical reactions. For example, it helps determine the feasibility of enzyme-catalyzed reactions.
-
Electrochemistry: ΔG° is directly related to the cell potential (E°) of an electrochemical cell, enabling the calculation of the maximum electrical work that can be obtained from a galvanic cell or the minimum energy required to drive an electrolytic cell.
-
Chemical Equilibrium: The relationship between ΔG° and K<sub>eq</sub> allows for the determination of equilibrium constants from thermodynamic data and vice versa.
-
Materials Science: Understanding ΔG° is crucial in predicting the stability and reactivity of materials, helping in the design of new materials with desired properties.
Conclusion
Calculating standard free energy change is a fundamental skill in chemistry and related fields. This guide has presented multiple methods for this calculation, emphasizing their underlying principles and offering practical examples. Understanding the relationship between ΔG°, enthalpy, entropy, temperature, and equilibrium constant is crucial for predicting the spontaneity of chemical and biochemical reactions and exploring their applications in various scientific and engineering disciplines. Remember to always consider the specific conditions, utilizing the appropriate equation for accurate calculations and meaningful interpretations of the results.
Latest Posts
Latest Posts
-
The Final Electron Acceptor Of Cellular Respiration Is
Apr 02, 2025
-
Monomers Are Connected In What Type Of Reaction
Apr 02, 2025
-
Find The Equation Of The Vertical Line
Apr 02, 2025
-
Electron Configuration For Copper And Chromium
Apr 02, 2025
-
Confidence Interval Calculator With Two Samples
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate Standard Free Energy Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
