A Neutral Atom Has Equal Numbers Of Blank And Electrons
Muz Play
Mar 23, 2025 · 5 min read
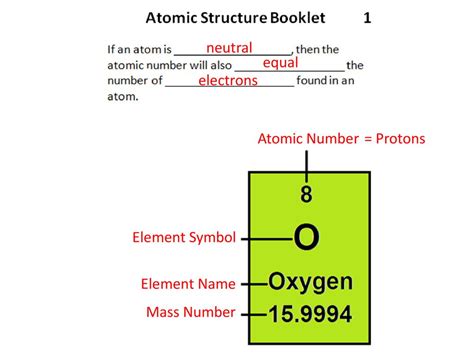
Table of Contents
A Neutral Atom Has Equal Numbers of Protons and Electrons: Understanding Atomic Structure and Charge
Atoms, the fundamental building blocks of matter, are incredibly complex entities. Understanding their structure is crucial to grasping the principles of chemistry and physics. A key concept in atomic structure is the relationship between protons, neutrons, and electrons, particularly in a neutral atom, where the number of protons and electrons are equal. This article will delve into this fundamental concept, exploring its implications and connections to other aspects of atomic theory.
The Basic Structure of an Atom
Atoms are composed of three primary subatomic particles:
- Protons: Positively charged particles found within the atom's nucleus.
- Neutrons: Neutrally charged particles also residing in the atom's nucleus.
- Electrons: Negatively charged particles that orbit the nucleus in electron shells or energy levels.
The nucleus, the atom's dense central core, contains both protons and neutrons. The number of protons in an atom's nucleus defines its atomic number, which uniquely identifies an element on the periodic table. For instance, hydrogen (H) has an atomic number of 1, meaning it has one proton in its nucleus. Oxygen (O) has an atomic number of 8, indicating eight protons.
Electrons, significantly lighter than protons and neutrons, are found in regions surrounding the nucleus called electron shells or energy levels. These shells are arranged in increasing energy levels, with electrons occupying the lowest energy levels first. The arrangement of electrons in these shells determines an atom's chemical properties and how it interacts with other atoms.
What Makes an Atom Neutral?
The key to understanding a neutral atom lies in the balance between its positive and negative charges. A proton carries a single positive charge (+1), while an electron carries a single negative charge (-1). Neutrons, as their name suggests, have no charge (0).
A neutral atom is an atom where the number of positively charged protons in the nucleus is exactly balanced by the number of negatively charged electrons orbiting the nucleus. This means the total positive charge of the protons is equal to the total negative charge of the electrons, resulting in a net charge of zero.
In a nutshell: A neutral atom has an equal number of protons and electrons.
This equality of protons and electrons is a fundamental principle of atomic structure. Any deviation from this balance results in an ion, which carries a net positive or negative charge.
Ions: When the Balance is Disturbed
When an atom loses or gains electrons, it becomes an ion.
-
Cations: If an atom loses electrons, it becomes a cation, carrying a net positive charge because the number of protons now exceeds the number of electrons. For example, a sodium atom (Na) can lose one electron to become a sodium ion (Na+), which has a +1 charge.
-
Anions: If an atom gains electrons, it becomes an anion, carrying a net negative charge because the number of electrons now exceeds the number of protons. For example, a chlorine atom (Cl) can gain one electron to become a chloride ion (Cl-), which has a -1 charge.
The formation of ions is a crucial process in many chemical reactions, enabling the formation of ionic compounds through electrostatic attraction between oppositely charged ions.
Isotopes: Variations in Neutron Number
While the number of protons defines the element, the number of neutrons can vary. Isotopes are atoms of the same element (same number of protons) but with different numbers of neutrons. This difference in neutron number affects the atom's mass but not its chemical properties significantly.
For example, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Both are isotopes of carbon, but ¹⁴C is radioactive.
The mass number of an atom is the sum of its protons and neutrons. Isotopes are often identified by their mass number.
The Significance of Equal Protons and Electrons in Chemical Reactions
The equal number of protons and electrons in a neutral atom is paramount to its chemical behavior. The electrons, particularly those in the outermost shell (valence electrons), are directly involved in chemical bonding. The arrangement of these valence electrons determines an atom's reactivity and how it interacts with other atoms to form molecules and compounds.
In chemical reactions, atoms may lose, gain, or share electrons to achieve a more stable electron configuration, often involving the filling of their outermost electron shell. This process is driven by the tendency of atoms to achieve a lower energy state, resulting in the formation of chemical bonds.
The neutrality of an atom implies a balance in its electrostatic forces. The positive charge of the nucleus is effectively shielded by the negative charge of the electrons, preventing strong electrostatic interactions with other atoms unless the electron distribution is altered (e.g., during bond formation).
Applications and Further Exploration
The concept of a neutral atom with equal numbers of protons and electrons is fundamental to many areas of science and technology:
-
Nuclear Chemistry: Understanding isotopes and their radioactive decay is critical in nuclear energy, medical imaging (PET scans), and carbon dating.
-
Analytical Chemistry: Techniques like mass spectrometry are used to determine the isotopic composition of samples, providing valuable information about their origin and composition.
-
Materials Science: The properties of materials are strongly influenced by the atomic structure and electron configuration of their constituent atoms.
-
Nanotechnology: Manipulating individual atoms and molecules requires a deep understanding of atomic structure and interactions.
Conclusion: A Cornerstone of Atomic Theory
The principle that a neutral atom possesses an equal number of protons and electrons is a cornerstone of atomic theory. This seemingly simple statement underpins our understanding of atomic structure, chemical bonding, and the behavior of matter at the atomic level. From the stability of individual atoms to the formation of complex molecules and materials, the balance between protons and electrons is essential. Further exploration into the intricacies of atomic structure continues to unlock new possibilities in science and technology, highlighting the enduring significance of this fundamental principle. Mastering this concept opens doors to a deeper understanding of the world around us, from the smallest particles to the largest structures. The implications of this seemingly simple idea are far-reaching and continue to inspire research and innovation across numerous scientific disciplines. The pursuit of knowledge at the atomic level is an ongoing journey, and understanding this foundational principle remains paramount to that quest.
Latest Posts
Latest Posts
-
Pronation And Supination Are Types Of Movements
Mar 25, 2025
-
Responsibilities And Rights Of A Citizen
Mar 25, 2025
-
What Is The Absolute Value Of 8
Mar 25, 2025
-
Which State Of Matter Takes The Shape Of Its Container
Mar 25, 2025
-
Difference Between Pulmonary Circulation And Systemic Circulation
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about A Neutral Atom Has Equal Numbers Of Blank And Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
