Which State Of Matter Takes The Shape Of Its Container
Muz Play
Mar 25, 2025 · 6 min read
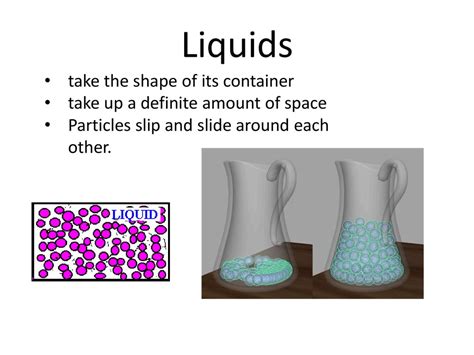
Table of Contents
Which State of Matter Takes the Shape of Its Container?
The question of which state of matter conforms to the shape of its container is a fundamental concept in chemistry and physics. While seemingly simple, understanding this property reveals deeper insights into the behavior of matter at a molecular level. This article delves into the characteristics of solids, liquids, and gases, explaining why only certain states readily adopt the form of their surroundings. We'll also explore the less common states of matter and their unique properties.
Understanding States of Matter
Before diving into the specifics, let's refresh our understanding of the three primary states of matter: solid, liquid, and gas. These states are distinguished by the arrangement and movement of their constituent particles (atoms, molecules, or ions).
Solids: Fixed Shape and Volume
Solids possess a definite shape and volume. Their particles are tightly packed in a highly ordered, rigid structure, held together by strong intermolecular forces. This strong attraction restricts the movement of particles, preventing them from flowing freely. As a result, solids resist any change in shape or volume. Think of a block of ice; it maintains its shape regardless of the container it's placed in.
Liquids: Fixed Volume, Variable Shape
Liquids have a fixed volume but take the shape of their container. Their particles are still close together, but the intermolecular forces are weaker than in solids, allowing for some movement and rearrangement. Liquids flow and conform to the contours of their vessel. Water in a glass takes the shape of the glass, but the amount of water (volume) remains constant. This adaptability distinguishes liquids from solids.
Gases: Variable Shape and Volume
Gases have neither a definite shape nor a definite volume; both are entirely determined by their container. The particles in gases are widely spaced, and the intermolecular forces are very weak. This allows particles to move freely and independently, resulting in gases readily filling any available space. Air fills a room, a balloon, or even a vacuum chamber— always conforming to the container's boundaries.
The Answer: Liquids and Gases
Therefore, the states of matter that take the shape of their containers are liquids and gases. Liquids exhibit this property due to the weaker intermolecular forces allowing their particles to move and rearrange themselves to fit the container's geometry. Gases, with their minimal intermolecular attraction, completely fill any available space, always mirroring the shape and volume of their container.
Deeper Dive into Liquid Behavior
The ability of liquids to conform to their container's shape is a consequence of their unique molecular properties. Several factors influence this adaptability:
Intermolecular Forces: The Glue that Holds (and Doesn't Hold) It Together
The strength of intermolecular forces plays a crucial role in a liquid's behavior. These forces are weaker than the intramolecular forces (bonds within molecules) but are strong enough to keep the molecules relatively close together, preventing them from dispersing like a gas. However, they're not strong enough to fix the molecules into a rigid structure, allowing for the liquid's fluidity and adaptability. Different types of intermolecular forces (hydrogen bonding, dipole-dipole interactions, London dispersion forces) lead to varying degrees of fluidity and viscosity.
Viscosity and Surface Tension: Influencing Shape Adaptation
The viscosity of a liquid, or its resistance to flow, influences how quickly it adapts to the shape of its container. High-viscosity liquids (like honey) flow more slowly and take longer to conform to the container's shape compared to low-viscosity liquids (like water). Surface tension, the tendency of liquid surfaces to minimize their area, also impacts shape adaptation. It creates a "skin" on the surface, influencing the liquid's meniscus (the curve at the liquid-air interface) in different containers.
Temperature and Pressure: Modifying Liquid Behavior
Temperature and pressure also impact a liquid's ability to conform to its container. Increasing temperature increases the kinetic energy of the molecules, weakening intermolecular forces and reducing viscosity, thus making the liquid adapt more readily. Similarly, changes in pressure can influence the volume of the liquid, indirectly affecting its shape within the container, although this effect is often less pronounced than temperature changes.
Gas Behavior and Container Adaptation
The behavior of gases in relation to their containers is governed by the kinetic theory of gases. This theory explains that gas particles are in constant, random motion, colliding with each other and the walls of their container. Several factors contribute to their complete filling of the container:
Kinetic Energy and Particle Motion
The high kinetic energy of gas particles enables them to overcome weak intermolecular forces, moving freely and independently. Their random motion ensures that they distribute themselves evenly throughout the entire available space.
Compressibility and Expansibility
Gases are highly compressible and expansible. Reducing the volume of the container compresses the gas, increasing its pressure. Conversely, expanding the container allows the gas to expand, reducing its pressure until it equally fills the new volume. This adaptability to volume changes directly leads to adaptation of shape.
Pressure and Temperature: Impact on Gas Distribution
Pressure and temperature are critical in governing gas behavior and its adaptation to container shape. Higher temperatures lead to increased kinetic energy, enhancing the particles' ability to fill the container. Higher pressures, conversely, increase the frequency of collisions between particles and the container walls, leading to a more even distribution within the available space. Gas laws (Boyle's Law, Charles' Law, Ideal Gas Law) mathematically describe these relationships.
Beyond the Three Primary States: Plasma and Bose-Einstein Condensates
While solids, liquids, and gases are the most commonly encountered states of matter, other states exist under specific conditions:
Plasma: Ionized Gas
Plasma is often considered the fourth state of matter. It is an ionized gas, meaning that some or all of its constituent atoms have lost or gained electrons, resulting in a mixture of ions and free electrons. Like gases, plasmas take the shape of their container, but their electrical conductivity and responsiveness to electromagnetic fields make their behavior significantly different from ordinary gases. Examples of plasma include lightning, the sun, and fluorescent lights.
Bose-Einstein Condensate: Supercooled Matter
At extremely low temperatures, near absolute zero, certain atoms can form a Bose-Einstein condensate (BEC). This state exhibits unique quantum mechanical properties, where a large fraction of the atoms occupy the lowest quantum state. While the BEC's behavior is governed by quantum mechanics rather than classical physics, it still adopts the shape of its container due to the extremely low kinetic energy of its particles.
Conclusion: Shape-Shifting Matter
In conclusion, liquids and gases are the states of matter that readily take the shape of their containers. This property stems from the differing strengths of intermolecular forces and the kinetic energy of their constituent particles. Liquids exhibit this through their ability to flow and rearrange, while gases, with their almost nonexistent intermolecular forces, completely fill the available space. Understanding this fundamental difference in behavior is essential for grasping the basic principles of chemistry and physics. Moreover, exploring less common states like plasma and Bose-Einstein condensates reveals the fascinating diversity and complexity of matter in various states, each with its unique properties and characteristics.
Latest Posts
Latest Posts
-
What Is A Subscript In A Chemical Equation
Mar 26, 2025
-
How To Do Post Closing Trial Balance
Mar 26, 2025
-
Como Multiplicar Dos Raices Cuadradas Dividas Entre Otra Raiz Cuadrada
Mar 26, 2025
-
Solid Liquid And Gas Elements In Periodic Table
Mar 26, 2025
-
Integration And Differentiation Of Power Series
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Which State Of Matter Takes The Shape Of Its Container . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
