What Is A Subscript In A Chemical Equation
Muz Play
Mar 26, 2025 · 6 min read
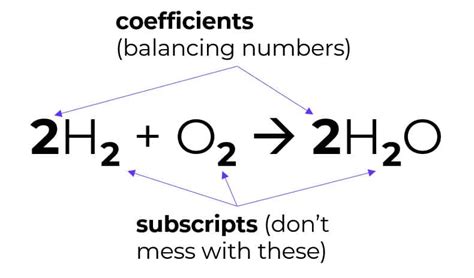
Table of Contents
What is a Subscript in a Chemical Equation? A Comprehensive Guide
Subscripts in chemical equations are not merely small numbers; they are fundamental components that convey crucial information about the composition and structure of molecules and compounds. Understanding their meaning is essential for anyone working with or studying chemistry. This comprehensive guide will delve into the intricacies of subscripts, explaining their significance and how they are used to represent chemical formulas and reactions.
Understanding Chemical Formulas and Subscripts
A chemical formula uses symbols and numbers to represent the composition of a substance. For instance, the formula for water is H₂O. This seemingly simple notation holds a wealth of information: it tells us that a water molecule consists of two hydrogen (H) atoms and one oxygen (O) atom. The crucial element here is the subscript '2' following the hydrogen symbol (H). This subscript indicates the number of hydrogen atoms present in each molecule of water.
Key takeaway: The subscript follows directly after the element symbol and applies only to that element. It is critical to understand that the subscript is not part of the element's symbol itself; it's a separate quantity signifying the number of atoms.
The Role of Subscripts in Representing Molecules
Subscripts are critical in differentiating between various chemical substances. Consider the following examples:
- H₂O (Water): Two hydrogen atoms, one oxygen atom.
- H₂O₂ (Hydrogen peroxide): Two hydrogen atoms, two oxygen atoms.
A seemingly small change in the subscript drastically alters the properties and characteristics of the substance. Water is essential for life, while hydrogen peroxide is a potent bleaching agent and antiseptic. This stark difference illustrates the importance of accurate subscript usage.
Subscripts in Ionic Compounds
Subscripts also play a vital role in representing ionic compounds, which are formed through the electrostatic attraction between oppositely charged ions (cations and anions). The subscripts in ionic compounds reflect the ratio of cations to anions needed to achieve electrical neutrality.
For example, consider sodium chloride (NaCl), common table salt. Sodium (Na) has a +1 charge, while chlorine (Cl) has a -1 charge. The formula NaCl implies a 1:1 ratio of sodium and chlorine ions, resulting in a neutral compound.
However, in compounds like magnesium chloride (MgCl₂), magnesium (Mg) has a +2 charge, while chlorine (Cl) still has a -1 charge. To balance the charges, two chlorine ions are needed for every magnesium ion, hence the subscript '2' on chlorine.
Understanding the charges of ions is crucial for correctly determining subscripts in ionic compounds.
Subscripts and Molecular Weight Calculations
Subscripts are fundamental in calculating the molecular weight (or molar mass) of a compound. The molecular weight is the sum of the atomic weights of all atoms present in a molecule.
To calculate the molecular weight of water (H₂O), you would:
- Look up the atomic weights of hydrogen (approximately 1 atomic mass unit or amu) and oxygen (approximately 16 amu).
- Multiply the atomic weight of hydrogen by its subscript (2): 1 amu/atom * 2 atoms = 2 amu.
- Add the atomic weight of oxygen: 2 amu + 16 amu = 18 amu.
Therefore, the molecular weight of water is approximately 18 amu. This calculation would be impossible without the subscript information.
Subscripts in Balanced Chemical Equations
Balanced chemical equations use subscripts to ensure that the number of atoms of each element is conserved throughout the reaction. The Law of Conservation of Mass dictates that matter cannot be created or destroyed in a chemical reaction; only rearranged. Therefore, the number of atoms of each element on the reactant side (left) must equal the number of atoms of the same element on the product side (right).
For example, consider the reaction between hydrogen and oxygen to form water:
2H₂ + O₂ → 2H₂O
The subscripts in this equation ensure the law of conservation of mass is obeyed. There are four hydrogen atoms and two oxygen atoms on both the reactant and product sides. The coefficient "2" in front of H₂ and H₂O are coefficients, indicating the number of molecules involved.
Subscripts vs. Coefficients: A Crucial Distinction
It's crucial to distinguish between subscripts and coefficients in chemical equations. Subscripts indicate the number of atoms of a particular element within a molecule, while coefficients indicate the number of molecules of a substance involved in the reaction. Mixing up these two is a common mistake that can lead to incorrect stoichiometric calculations.
Common Mistakes and Misconceptions Regarding Subscripts
Several common errors occur when dealing with subscripts:
- Misinterpreting subscripts as coefficients: This leads to inaccurate calculations of molecular weights and unbalanced chemical equations.
- Incorrectly writing subscripts: Forgetting subscripts or placing them incorrectly can significantly change the meaning of the chemical formula.
- Ignoring subscripts in calculations: Leaving out subscripts in stoichiometry calculations can lead to erroneous results.
Careful attention to detail and a clear understanding of the function of subscripts are paramount in avoiding these errors.
Advanced Applications of Subscripts
Beyond the basics, subscripts are involved in more advanced concepts:
- Polyatomic ions: Subscripts within parentheses indicate the number of polyatomic ions present in a compound. For example, in Ca(NO₃)₂, the subscript '2' indicates two nitrate (NO₃) ions are present.
- Hydrates: Subscripts are used to represent the number of water molecules associated with a compound in a hydrate. For example, CuSO₄·5H₂O indicates that five water molecules are associated with each copper sulfate molecule.
- Isomers: While subscripts do not directly distinguish between isomers (molecules with the same molecular formula but different arrangements of atoms), understanding the molecular formula from subscripts provides a basis for identifying and analyzing isomers.
Practical Exercises to Master Subscripts
To solidify your understanding, try these exercises:
- Determine the molecular weight of various compounds, given their chemical formulas (e.g., CO₂, CH₄, NaCl).
- Write balanced chemical equations for simple reactions, ensuring correct subscript usage.
- Identify the number of atoms of each element present in a given chemical formula.
- Practice differentiating between subscripts and coefficients.
Conclusion
Subscripts are a fundamental aspect of chemical notation. They convey critical information about molecular composition, enable accurate calculations of molecular weights, and ensure the balancing of chemical equations. Understanding subscripts is a cornerstone of mastering fundamental chemistry concepts and venturing into more advanced topics. Careful attention to subscripts and their distinction from coefficients is essential for accuracy and success in any chemical endeavor. Mastering this fundamental concept will pave the way for a deeper comprehension of the intricacies of chemical reactions and the structure of matter itself. By diligently practicing and applying the concepts discussed here, you can build a solid foundation in chemistry and confidently approach more complex chemical problems.
Latest Posts
Latest Posts
-
Where Is The Bacterial Chromosome Located
Mar 29, 2025
-
Write The Chemical Formula For This Molecule
Mar 29, 2025
-
How To Calculate Velocity From Flow Rate
Mar 29, 2025
-
Write The Iupac Names Of The Given Carboxylic Acids
Mar 29, 2025
-
Multiplication Of A Polynomial By A Monomial
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is A Subscript In A Chemical Equation . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
