Write The Chemical Formula For This Molecule
Muz Play
Mar 29, 2025 · 5 min read
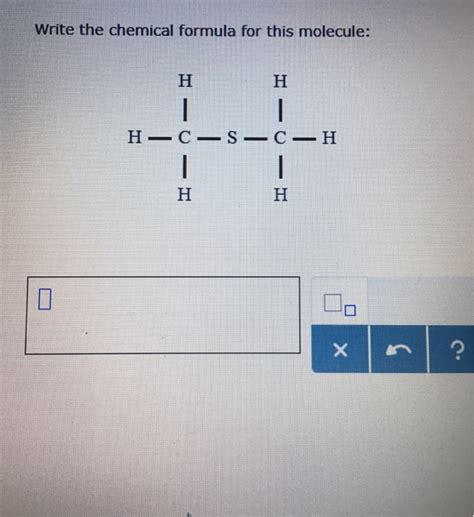
Table of Contents
Write the Chemical Formula for This Molecule: A Comprehensive Guide
Determining the chemical formula for a molecule is a fundamental task in chemistry. This process, seemingly simple at first glance, requires a solid understanding of molecular structure, bonding, and nomenclature. This article will delve deep into the various methods and considerations involved in writing the chemical formula for any given molecule, from simple diatomic molecules to complex organic compounds. We'll cover everything from basic principles to advanced techniques, ensuring a comprehensive understanding for both beginners and experienced learners.
Understanding Chemical Formulas
Before we dive into the specifics, let's clarify what a chemical formula represents. A chemical formula is a concise way of representing the number and type of atoms present in a molecule. It uses chemical symbols (e.g., H for hydrogen, O for oxygen, C for carbon) and subscripts to indicate the number of each atom.
For example:
- H₂O: This represents a water molecule, containing two hydrogen atoms and one oxygen atom.
- CO₂: This represents a carbon dioxide molecule, containing one carbon atom and two oxygen atoms.
- C₆H₁₂O₆: This represents a glucose molecule, a more complex molecule with six carbon atoms, twelve hydrogen atoms, and six oxygen atoms.
There are several types of chemical formulas:
- Empirical Formula: This shows the simplest whole-number ratio of atoms in a compound. For example, the empirical formula for glucose (C₆H₁₂O₆) is CH₂O.
- Molecular Formula: This shows the actual number of atoms of each element in a molecule. For glucose, the molecular formula is C₆H₁₂O₆.
- Structural Formula: This shows not only the number and type of atoms but also their arrangement within the molecule, using lines to represent bonds. Structural formulas are crucial for understanding the properties and reactivity of molecules, especially organic ones.
- Condensed Structural Formula: A simplified version of the structural formula, often used for organic molecules to save space. It shows the connectivity of atoms but doesn't explicitly draw all the bonds.
Determining Chemical Formulas: Step-by-Step Guide
The process of determining the chemical formula depends on the information available. Let's explore different scenarios:
1. Using the Name of the Compound
For simple inorganic compounds, the name often directly indicates the elements and their ratios. This is particularly true for binary compounds (compounds with two elements).
Example: Sodium chloride (NaCl)
The name clearly indicates the presence of sodium (Na) and chlorine (Cl) in a 1:1 ratio.
For more complex inorganic compounds, understanding prefixes and oxidation states becomes crucial.
Example: Dinitrogen pentoxide (N₂O₅)
The prefixes "di" and "pent" indicate two nitrogen atoms and five oxygen atoms, respectively.
2. Using the Molecular Structure
If you have the molecular structure (either a drawing or a 3D model), counting the number of each type of atom is straightforward.
Example: Consider a molecule of methane (CH₄). The structural formula clearly shows one carbon atom bonded to four hydrogen atoms. Therefore, the chemical formula is CH₄.
3. Using Experimental Data (Percent Composition and Molar Mass)
This is a more advanced method, often used in analytical chemistry. If you know the percent composition of each element in a compound and its molar mass, you can determine the empirical and molecular formulas.
Steps:
- Assume a 100g sample: This simplifies the calculations because the percentages directly translate to grams.
- Convert grams to moles: Divide the mass of each element by its molar mass (found on the periodic table).
- Determine the mole ratio: Divide the number of moles of each element by the smallest number of moles obtained in step 2. This gives you the simplest whole-number ratio of atoms. This is the empirical formula.
- Determine the molecular formula: If you know the molar mass of the compound, divide the molar mass by the empirical formula mass. This gives you a whole number (n). Multiply the subscripts in the empirical formula by 'n' to obtain the molecular formula.
Example: A compound is found to contain 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen. Its molar mass is 60.0 g/mol.
- 100g sample: 40.0g C, 6.7g H, 53.3g O
- Moles:
- C: 40.0g / 12.01 g/mol = 3.33 mol
- H: 6.7g / 1.01 g/mol = 6.63 mol
- O: 53.3g / 16.00 g/mol = 3.33 mol
- Mole ratio:
- C: 3.33 mol / 3.33 mol = 1
- H: 6.63 mol / 3.33 mol ≈ 2
- O: 3.33 mol / 3.33 mol = 1
- Empirical formula: CH₂O
- Molecular formula:
- Empirical formula mass: 12.01 + 2(1.01) + 16.00 = 30.03 g/mol
- n = 60.0 g/mol / 30.03 g/mol ≈ 2
- Molecular formula: C₂H₄O₂
Advanced Considerations: Isomerism and Complex Molecules
For more complex molecules, especially organic compounds, isomerism plays a crucial role. Isomers are molecules with the same molecular formula but different structural arrangements. Therefore, simply knowing the chemical formula doesn't fully describe the molecule; you need the structural formula to differentiate between isomers.
Example: C₂H₆O can represent either ethanol (CH₃CH₂OH) or dimethyl ether (CH₃OCH₃), which have very different properties.
Determining the chemical formula for macromolecules like proteins and polymers requires advanced techniques like mass spectrometry and various spectroscopic methods (NMR, IR, etc.). These techniques provide information about the composition and arrangement of atoms, allowing for the determination of the chemical formula and structure.
Conclusion: Mastering Chemical Formulas
Writing the chemical formula for a molecule is a fundamental skill in chemistry. This comprehensive guide has covered various methods, from simple name-based deduction to more complex analyses involving experimental data. Understanding the different types of chemical formulas – empirical, molecular, structural, and condensed structural – is crucial for accurately representing molecules and their properties. While simple molecules yield easily to straightforward approaches, complex molecules and isomers necessitate a deeper understanding of molecular structure and advanced analytical techniques. Mastering these concepts is essential for success in chemistry and related fields. Remember to always consider the context and available information when determining the chemical formula. By applying the methods discussed here and further exploring the vast world of chemical analysis, you'll gain a robust skillset to confidently determine the chemical formula for any molecule you encounter.
Latest Posts
Latest Posts
-
What Is The Basic Structural Unit Of The Body
Apr 01, 2025
-
Motion Of Molecules Compared To Direction Of Motion Electromagnetic Waves
Apr 01, 2025
-
The Variance Is The Square Root Of The Standard Deviation
Apr 01, 2025
-
Easy Way To Find Common Multiples
Apr 01, 2025
-
What Is The Structural And Functional Unit Of The Kidney
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Write The Chemical Formula For This Molecule . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
