Write The Iupac Names Of The Given Carboxylic Acids
Muz Play
Mar 31, 2025 · 6 min read
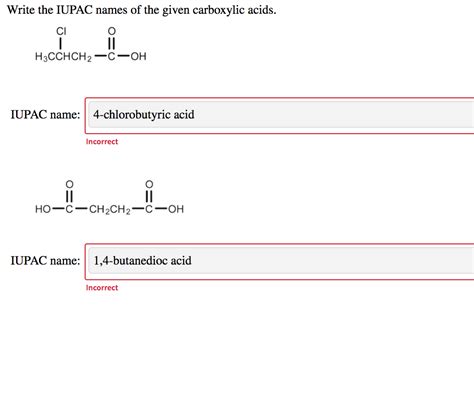
Table of Contents
- Write The Iupac Names Of The Given Carboxylic Acids
- Table of Contents
- IUPAC Nomenclature of Carboxylic Acids: A Comprehensive Guide
- Understanding the Basic Structure and Functional Group
- Systematic IUPAC Naming: A Step-by-Step Approach
- 1. Identifying the Parent Chain
- 2. Numbering the Carbon Chain
- 3. Identifying and Naming Substituents
- 4. Combining the Names
- Illustrative Examples: Building Your Understanding
- Dealing with More Complex Structures
- Common vs. IUPAC Names: A Note of Caution
- Beyond the Basics: Advanced Cases
- Unsaturated Carboxylic Acids
- Cyclic Carboxylic Acids
- Polycarboxylic Acids
- Aromatic Carboxylic Acids
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
IUPAC Nomenclature of Carboxylic Acids: A Comprehensive Guide
Carboxylic acids, characterized by the presence of a carboxyl group (-COOH), form a fundamental class of organic compounds. Understanding their IUPAC nomenclature is crucial for effective communication and comprehension in organic chemistry. This comprehensive guide will delve into the systematic naming of carboxylic acids, covering various complexities and providing numerous examples to solidify your understanding.
Understanding the Basic Structure and Functional Group
Before diving into the intricacies of naming, let's establish a firm grasp on the basic structure of carboxylic acids. The core of a carboxylic acid is the carboxyl group, a combination of a carbonyl group (C=O) and a hydroxyl group (-OH) bonded to the same carbon atom. This carbon atom is always considered carbon number 1 (C1) in the parent chain. The general formula for a carboxylic acid is R-COOH, where R represents any alkyl or aryl group.
Systematic IUPAC Naming: A Step-by-Step Approach
The IUPAC system offers a logical and unambiguous method for naming carboxylic acids. Here's a breakdown of the process:
1. Identifying the Parent Chain
The parent chain is the longest continuous carbon chain containing the carboxyl group. The carboxyl carbon (C=O-OH) is always included in this chain.
Example: Consider the molecule CH₃CH₂CH₂COOH. The longest chain containing the carboxyl group has four carbons.
2. Numbering the Carbon Chain
The carbon chain is numbered starting from the carboxyl carbon (C1). Numbering proceeds in the direction that gives the substituents the lowest possible numbers.
Example: In CH₃CH₂CH₂COOH, the carboxyl carbon is C1, followed by C2, C3, and C4.
3. Identifying and Naming Substituents
Substituents are any atoms or groups attached to the parent chain other than the carboxyl group. These are named using standard IUPAC rules. Their positions are indicated by the number of the carbon atom to which they are attached.
Example: Consider CH₃CH(CH₃)CH₂COOH. A methyl group (-CH₃) is attached to C2.
4. Combining the Names
The name is constructed by:
- Prefixes: These indicate the substituents and their positions. For example, 2-methyl indicates a methyl group attached to carbon 2.
- Parent Chain Name: This is derived from the number of carbons in the parent chain (e.g., butanoic acid for a four-carbon chain). The "-oic acid" suffix denotes the carboxylic acid functional group.
Example: CH₃CH(CH₃)CH₂COOH is named 2-methylbutanoic acid.
Illustrative Examples: Building Your Understanding
Let's work through several examples to illustrate the application of IUPAC naming rules:
-
Example 1: CH₃COOH (Acetic Acid) – This is the simplest carboxylic acid. The parent chain has two carbons. The name is ethanoic acid. (Common name: Acetic acid)
-
Example 2: CH₃CH₂CH₂CH₂COOH (Pentanoic Acid) – The parent chain has five carbons. The name is pentanoic acid. (Common name: Valeric acid)
-
Example 3: CH₃CH₂CH(CH₃)COOH (2-Methylbutanoic Acid) – The parent chain has four carbons, with a methyl substituent on carbon 2. The name is 2-methylbutanoic acid. (Common name: Isovaleric acid)
-
Example 4: (CH₃)₂CHCH₂COOH (3-Methylbutanoic Acid) – The longest carbon chain is four carbons long. A methyl group is located at position 3. The name is 3-methylbutanoic acid. (Common name: Isovaleric acid - Note: this highlights that common names may sometimes not be unique)
-
Example 5: CH₃CH₂CH(Cl)CH₂COOH (4-chloropentanoic acid) – A chlorine atom is a substituent. The name is 4-chloropentanoic acid.
-
Example 6: HOOC-CH₂-CH₂-COOH (Butanedioic acid) - This molecule contains two carboxylic acid groups. The suffix "-dioic acid" is used when multiple carboxylic acid groups are present. (Common name: Succinic acid)
-
Example 7: HOOC-(CH₂)₄-COOH (Hexanedioic acid) - Similarly, this has two carboxyl groups, but the parent chain is six carbons long.
-
Example 8: CH₃CH(CH₂CH₃)CH₂COOH (3-Methylpentanoic acid) – Notice how the longest chain containing the carboxyl group is chosen, even if it means a longer substituent.
-
Example 9: CH₃CH(COOH)CH₂CH₃ (2-Methylbutanoic acid) – The numbering starts from the carboxyl carbon regardless of where it is placed on the chain.
-
Example 10: ClCH₂CH₂CH₂COOH (4-chlorobutanoic acid) - The position of the chlorine atom is specified correctly, indicating its placement on the fourth carbon atom.
Dealing with More Complex Structures
The naming principles remain the same, even with more complex structures. The key is to systematically identify the longest carbon chain containing the carboxyl group, number the chain, and name all substituents. Remember to always use the lowest possible numbers for substituent positions.
For molecules with multiple functional groups, prioritize the carboxylic acid group. Other functional groups will be named as substituents. For instance, if a hydroxyl group (-OH) and a carboxyl group are present in the same molecule, the molecule is named as a hydroxycarboxylic acid. The position of the hydroxyl group is indicated by a number.
Common vs. IUPAC Names: A Note of Caution
While IUPAC names provide unambiguous identification, common names are often used in practice, particularly for simpler carboxylic acids. For instance, CH₃COOH is commonly known as acetic acid, while its IUPAC name is ethanoic acid. While using common names might be convenient in certain contexts, it's essential to be aware that they lack the systematic consistency of IUPAC names and can sometimes be ambiguous. Therefore, for clarity and scientific accuracy, IUPAC nomenclature is preferred, especially when dealing with complex molecules.
Beyond the Basics: Advanced Cases
Unsaturated Carboxylic Acids
Unsaturated carboxylic acids contain carbon-carbon double or triple bonds. The position of the double or triple bond is indicated by a number, and the suffix "-enoic acid" or "-ynoic acid" is used, respectively.
- Example: CH₂=CHCOOH is propenoic acid.
Cyclic Carboxylic Acids
Cyclic carboxylic acids have the carboxyl group attached to a ring. The numbering starts from the carboxyl carbon, and the ring is named accordingly.
- Example: A cyclohexane ring with a carboxylic acid group would be cyclohexanecarboxylic acid.
Polycarboxylic Acids
Molecules with multiple carboxyl groups are named using the suffix "-dioic acid," "-trioic acid," etc., depending on the number of carboxyl groups.
- Example: HOOC-COOH is ethanedioic acid.
Aromatic Carboxylic Acids
Aromatic carboxylic acids have the carboxyl group attached to an aromatic ring (e.g., benzene ring). The carboxyl group is considered a substituent, and the name is derived from the aromatic ring.
- Example: Benzoic acid is the simplest aromatic carboxylic acid.
Conclusion
Mastering the IUPAC nomenclature of carboxylic acids is a fundamental skill for any student or professional working in organic chemistry. This guide has provided a thorough overview of the naming conventions, from basic structures to more complex cases. By diligently practicing with examples and working through different structures, you will develop a solid understanding and confidence in applying these essential naming rules. Remember the systematic approach and prioritized numbering systems are key to accurately and efficiently naming even the most complex carboxylic acid structures. Consistent practice and attention to detail are the best methods for mastering this valuable skill.
Latest Posts
Latest Posts
-
Clonal Selection Of T Cells Happens In The Thymus
Apr 01, 2025
-
Formula Para Sacar Diametro De Un Circulo
Apr 01, 2025
-
How To Make A Frequency Histogram In Excel
Apr 01, 2025
-
What Is The Monomer Of A Dna Molecule
Apr 01, 2025
-
When Does The Segregation Of Alleles Occur
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Write The Iupac Names Of The Given Carboxylic Acids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
