Solid Liquid And Gas Elements In Periodic Table
Muz Play
Mar 26, 2025 · 5 min read
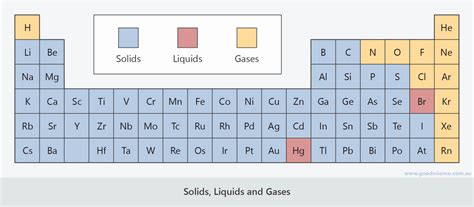
Table of Contents
Solid, Liquid, and Gas Elements in the Periodic Table: A Comprehensive Overview
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While the table beautifully illustrates trends in electronegativity, ionization energy, and atomic radius, it doesn't explicitly depict the physical state of elements at standard temperature and pressure (STP). However, understanding the relationship between an element's position on the table and its physical state at STP provides crucial insights into its properties and behavior. This article delves into the fascinating world of solid, liquid, and gaseous elements, exploring their distribution within the periodic table, and examining the underlying factors that dictate their physical states.
The Prevalence of Solid Elements
The vast majority of elements in the periodic table exist as solids at STP. This isn't a coincidence; the strong interatomic forces within these elements dictate their solid nature. These forces vary, from metallic bonding in metals to covalent networks in nonmetals, and ionic interactions in salts.
Metallic Solids: A Sea of Electrons
Most metallic elements are solids at STP, forming a dense, closely packed structure. This is due to metallic bonding, where valence electrons are delocalized, forming a "sea" of electrons that surrounds positively charged metal ions. This "electron sea" allows for excellent electrical and thermal conductivity, malleability (ability to be hammered into sheets), and ductility (ability to be drawn into wires). This is why metals like iron (Fe), copper (Cu), gold (Au), and silver (Ag) are prominent examples of solid metallic elements. Their positions, primarily towards the left of the periodic table, reflect their tendency towards losing electrons to achieve a stable configuration.
Covalent Network Solids: Strong Bonds, High Melting Points
Some non-metallic elements form covalent network solids. These solids are characterized by a continuous network of covalent bonds between atoms, leading to incredibly strong and rigid structures. Consequently, these elements boast exceptionally high melting and boiling points. Diamond (a form of carbon, C), silicon (Si), and boron (B) are prime examples. Their structures are three-dimensional networks of strong covalent bonds, making them exceptionally hard and resistant to breakage. The high strength of these bonds requires significant energy to break, explaining their solid state at STP.
Ionic Solids: Electrostatic Attraction
Ionic compounds, formed by the electrostatic attraction between positively and negatively charged ions, predominantly exist as solids at STP. These ions are held together by strong Coulombic forces, resulting in crystalline structures with high melting points. Sodium chloride (NaCl), commonly known as table salt, is a perfect example. The strong attraction between Na⁺ and Cl⁻ ions creates a highly stable crystal lattice. Many other ionic compounds, featuring metals from groups 1 and 2 combined with nonmetals from groups 16 and 17, display similar solid-state properties at STP. The location of these elements on the periodic table reflects their tendencies to either readily lose or gain electrons to achieve a noble gas configuration.
The Rarity of Liquid Elements
At STP, only bromine (Br) and mercury (Hg) exist as liquids. This rarity highlights the specific conditions required for a liquid state.
Bromine: A Non-Metallic Liquid
Bromine, a halogen located in Group 17, exists as a reddish-brown liquid at STP. Its relatively weak intermolecular forces, London dispersion forces, compared to the strong covalent bonds in other halogens like chlorine and iodine (which exist as gases and solids, respectively at STP), allow it to remain liquid at normal temperatures. The larger size of bromine atoms compared to its lighter counterparts also plays a role in this lower boiling point.
Mercury: The Unique Liquid Metal
Mercury, a transition metal in Group 12, is unique in its liquid state at STP. While metallic bonding is present, the weak interatomic forces between mercury atoms, potentially due to the electron configuration of mercury resulting in weak metallic bonds, contribute to its low melting point. This characteristic distinguishes it from other transition metals, most of which are solid at STP. Furthermore, the strong relativistic effects in mercury further contribute to its unusual properties.
The World of Gaseous Elements
A significant number of elements exist as gases at STP. These gases, generally nonmetals, are characterized by weak intermolecular forces.
Noble Gases: Inert and Gaseous
The noble gases (He, Ne, Ar, Kr, Xe, Rn), located in Group 18, are all gases at STP. Their full valence electron shells result in extremely low reactivity and weak intermolecular forces. This explains their existence as monatomic gases, not forming molecules or significant interatomic bonds, at STP. Their stability dictates their gaseous state under normal conditions.
Diatomic Gases: Sharing Electrons
Several nonmetals exist as diatomic gases at STP. These include hydrogen (H₂), nitrogen (N₂), oxygen (O₂), fluorine (F₂), and chlorine (Cl₂). They form diatomic molecules because forming a covalent bond completes their outer electron shells, improving stability. The relatively weak intermolecular forces between these diatomic molecules allow for their existence as gases at STP.
Other Gaseous Elements
Other elements like phosphorus (P4), which exists as tetratomic molecules, and sulfur (S₈), which exists as octatomic molecules, are notable exceptions. While they are nonmetals, their stronger intermolecular forces compared to noble gases and diatomic molecules influence their ability to be gases.
Factors Influencing the Physical State
Several factors influence an element's physical state at STP:
- Atomic Mass and Size: Larger atoms generally have stronger London dispersion forces, leading to higher melting and boiling points.
- Intermolecular Forces: Strong intermolecular forces result in solids, while weaker forces favor liquids or gases.
- Bonding Type: Metallic bonding usually leads to solids, while weak intermolecular forces result in gases.
- Electron Configuration: Full valence shells (as in noble gases) lead to low reactivity and gaseous states.
Conclusion: A Dynamic Periodic Table
The periodic table provides a framework for understanding elemental properties, including their physical state. The prevalence of solid elements reflects the strength of metallic, covalent, and ionic bonds. The rarity of liquid elements emphasizes the specific conditions required for a liquid state at STP. Finally, the significant number of gaseous elements highlights the impact of weak intermolecular forces on physical state. While the periodic table itself doesn't directly indicate the physical state, understanding its organization and the underlying factors governing interatomic and intermolecular forces illuminates the diverse states of matter in which elements can exist, offering a deeper appreciation of the dynamic nature of the periodic system. Further research into the properties of elements and the forces governing their behavior continues to refine our understanding of this fundamental classification system.
Latest Posts
Latest Posts
-
Where Is The Bacterial Chromosome Located
Mar 29, 2025
-
Write The Chemical Formula For This Molecule
Mar 29, 2025
-
How To Calculate Velocity From Flow Rate
Mar 29, 2025
-
Write The Iupac Names Of The Given Carboxylic Acids
Mar 29, 2025
-
Multiplication Of A Polynomial By A Monomial
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Solid Liquid And Gas Elements In Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
