A Particle That Has A Negative Charge
Muz Play
Mar 18, 2025 · 6 min read
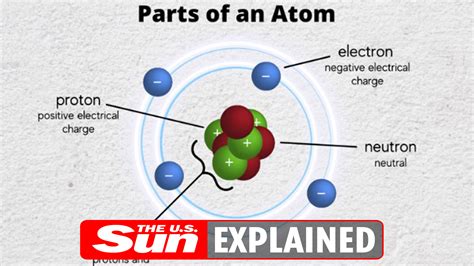
Table of Contents
A Particle That Has a Negative Charge: Delving into the World of Electrons
The universe is a vast and complex tapestry woven from fundamental building blocks. Among these, particles carrying a negative electric charge play a pivotal role in shaping the world as we know it. While several particles exhibit negative charge, the most prominent and readily understood is the electron. This article will delve deep into the fascinating world of electrons, exploring their properties, behaviors, and significance in various fields of science and technology.
Understanding the Electron: A Fundamental Particle
The electron, denoted by the symbol e⁻, is a fundamental subatomic particle with a negative elementary electric charge of −1.602 × 10⁻¹⁹ coulombs. Its mass is relatively tiny compared to protons and neutrons, approximately 1/1836 the mass of a proton. This minuscule mass contributes significantly to its behavior and interactions. Critically, electrons are considered fundamental particles, meaning they are not composed of smaller constituents. This stands in contrast to protons and neutrons, which are made up of quarks.
Key Properties of Electrons:
- Charge: -1 (elementary charge)
- Mass: 9.109 × 10⁻³¹ kg
- Spin: ½ (fermion)
- Size: Point-like (no measurable size)
The electron's spin of ½ classifies it as a fermion, a type of particle that obeys the Pauli Exclusion Principle. This principle dictates that no two electrons within an atom can occupy the same quantum state simultaneously. This is crucial for understanding the structure of atoms and the periodic table of elements. The "point-like" nature indicates that, to our current understanding, electrons have no measurable size; they are considered fundamental point particles.
The Role of Electrons in Atomic Structure
Electrons are key players in determining the chemical properties of atoms. They orbit the atom's nucleus, which contains positively charged protons and neutral neutrons. The number of electrons in an atom determines its overall charge and how it interacts with other atoms. A neutral atom has an equal number of protons and electrons. The arrangement of electrons in different energy levels or shells around the nucleus determines the atom's chemical reactivity and its position in the periodic table.
Electron Shells and Energy Levels:
Electrons reside in specific energy levels or shells surrounding the nucleus. These shells are characterized by distinct energy values, with electrons in lower shells possessing lower energy. The filling of electron shells dictates the atom's chemical behavior. Atoms strive for a stable electron configuration, often achieving this by gaining, losing, or sharing electrons with other atoms to complete their outermost electron shell (valence shell). This drive for stability underlies the formation of chemical bonds.
Chemical Bonding and Electron Interactions
The interaction between electrons from different atoms drives the formation of chemical bonds, holding molecules together. There are several types of chemical bonds, each involving distinct electron interactions:
Covalent Bonds:
In covalent bonds, atoms share electrons to achieve a stable electron configuration. This sharing creates a strong bond between the atoms. Many organic molecules, such as those that form the basis of life, are held together by covalent bonds. The strength and properties of the bond depend on the number of shared electrons and the electronegativity of the atoms involved.
Ionic Bonds:
Ionic bonds form when one atom transfers one or more electrons to another atom. This transfer creates ions: positively charged cations (atoms that lost electrons) and negatively charged anions (atoms that gained electrons). The electrostatic attraction between the oppositely charged ions forms the ionic bond. Table salt (NaCl) is a classic example of an ionic compound.
Metallic Bonds:
Metallic bonds are characterized by the delocalization of electrons among a lattice of metal atoms. These freely moving electrons are responsible for the characteristic properties of metals, such as electrical and thermal conductivity, malleability, and ductility.
Electrons in Electricity and Electronics
The movement of electrons is the fundamental principle behind electricity. Electric current is the flow of charge, and in most cases, this charge is carried by electrons. Our modern world relies heavily on the ability to control and harness the flow of electrons.
Electric Current and Conduction:
In conductors, such as metals, electrons are relatively free to move. An applied electric field causes these electrons to drift, creating an electric current. Insulators, on the other hand, hold their electrons tightly, preventing significant electron flow. Semiconductors occupy a middle ground, exhibiting controlled electrical conductivity that is crucial for modern electronics.
Semiconductor Devices and Electronics:
Semiconductor materials like silicon are the foundation of modern electronics. By carefully controlling the movement of electrons within semiconductors using doping (introducing impurities), we can create transistors, diodes, and integrated circuits. These components are the building blocks of computers, smartphones, and countless other electronic devices.
Electrons in Other Phenomena
Electrons' significance extends far beyond their roles in chemistry and electronics. They play vital roles in various other natural phenomena and technological applications:
Cathode Rays and Televisions:
Historically, the discovery of electrons was linked to the observation of cathode rays. These rays, streams of electrons emitted from a heated cathode, were crucial in early experiments that led to understanding the electron's properties. This same principle is used in older cathode ray tube (CRT) televisions.
Electron Microscopes:
Electron microscopes utilize beams of electrons to visualize objects at far higher resolutions than optical microscopes. The shorter wavelength of electrons allows for the imaging of much smaller structures, revolutionizing fields like materials science and biology.
Particle Accelerators:
Particle accelerators, such as the Large Hadron Collider, accelerate electrons to incredibly high speeds. By colliding these high-energy electrons with other particles, physicists can explore the fundamental laws of physics and discover new particles.
X-rays and Medical Imaging:
X-rays are produced when high-energy electrons are suddenly decelerated. This process emits electromagnetic radiation that can penetrate soft tissues but is absorbed by denser materials like bone. This property allows for the creation of X-ray images used extensively in medical diagnostics.
The Ongoing Study of Electrons
Despite centuries of research, the electron continues to be a subject of active study. Researchers are constantly pushing the boundaries of our understanding, exploring its fundamental properties and its role in complex phenomena. Some current areas of research include:
Quantum Computing:
Electrons' quantum properties, such as superposition and entanglement, are central to the development of quantum computers. These computers have the potential to solve problems that are intractable for classical computers.
Electron Spintronics:
Spintronics utilizes the electron's spin, rather than just its charge, to create new electronic devices. This field promises to lead to faster, more energy-efficient electronics.
Novel Materials and Nanomaterials:
The manipulation of electrons at the nanoscale is leading to the development of new materials with unique properties. These materials have applications in various fields, including medicine, energy, and electronics.
Conclusion: The Ubiquitous Electron
The electron, a tiny particle carrying a negative charge, is a fundamental component of matter and a cornerstone of our modern technological world. From the structure of atoms to the operation of sophisticated electronic devices, electrons play an integral role in shaping the universe and our understanding of it. Ongoing research promises even deeper insights into this fascinating particle, potentially unlocking new technologies and transforming our world in unforeseen ways. The enduring mystery and immense potential of the electron solidify its place as one of the most significant discoveries in the history of science. The continuous exploration of its properties and interactions continues to push the boundaries of scientific knowledge and technological advancement. Its influence permeates nearly every aspect of our modern lives, making it a truly ubiquitous and remarkable particle.
Latest Posts
Latest Posts
-
What Is The Total Magnification Of 4x
Mar 18, 2025
-
The Cutaneous Membrane Is Blank To The Muscles
Mar 18, 2025
-
If A Hybrid Offspring Does Not Survive
Mar 18, 2025
-
5 Postulates Of Daltons Atomic Theory
Mar 18, 2025
-
How To Find Linear Relationship Between Independent And Dependent Variables
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about A Particle That Has A Negative Charge . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
