5 Postulates Of Dalton's Atomic Theory
Muz Play
Mar 18, 2025 · 6 min read
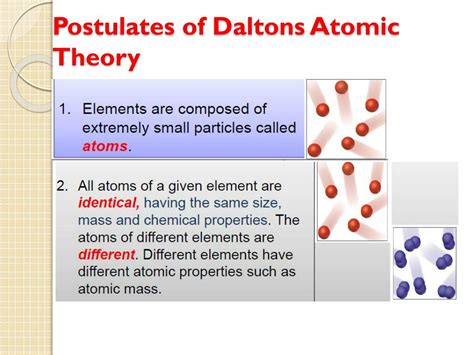
Table of Contents
5 Postulates of Dalton's Atomic Theory: A Comprehensive Look
John Dalton's atomic theory, proposed in the early 1800s, revolutionized our understanding of matter. While some aspects have been refined or superseded by modern quantum mechanics, its core principles remain foundational to chemistry. This article delves deep into Dalton's five postulates, exploring their significance, limitations, and enduring impact on scientific thought.
Postulate 1: All Matter is Made of Atoms
This seemingly simple statement was, at the time, a radical departure from existing beliefs. Dalton posited that all matter, regardless of its form – solid, liquid, or gas – is composed of indivisible and indestructible particles called atoms. This was a powerful concept, shifting the focus from continuous, infinitely divisible matter to a discrete, particulate model. Before Dalton, the prevailing view leaned toward philosophical concepts rather than a concrete, experimentally testable model.
Significance: This postulate laid the groundwork for a quantifiable understanding of matter. Instead of vague notions about substances, chemists now had a fundamental unit – the atom – to work with. This allowed for the development of stoichiometry, the study of quantitative relationships between reactants and products in chemical reactions.
Limitations: Modern science has proven that atoms are not indivisible. They are composed of subatomic particles – protons, neutrons, and electrons – which can be further broken down into even more fundamental particles. Nuclear reactions demonstrate the destruction and creation of atoms. However, in the context of chemical reactions, Dalton's postulate remains largely valid. Atoms are not created or destroyed in typical chemical processes; they merely rearrange themselves to form new compounds.
Postulate 2: All Atoms of a Given Element are Identical in Mass and Properties
This postulate establishes the concept of elemental identity at the atomic level. All atoms of a specific element, like gold (Au) or oxygen (O), possess the same mass and the same set of properties. This uniformity is crucial for understanding the consistent behavior of elements in chemical reactions.
Significance: This postulate introduced the idea of a standardized atomic unit for each element. It provided a framework for understanding why samples of the same element always exhibit the same characteristics. For example, all samples of pure gold have the same density, melting point, and reactivity.
Limitations: This postulate is not entirely accurate. The existence of isotopes – atoms of the same element with different numbers of neutrons – disproves this. Isotopes have the same number of protons (defining the element), but vary in neutron count, resulting in different atomic masses. While isotopes share chemical properties, their physical properties (like mass) can differ slightly. However, the difference in properties between isotopes of the same element is often negligible for many chemical applications.
Postulate 3: Atoms of Different Elements Differ in Mass and Properties
This postulate distinguishes between different types of atoms. The atoms of each element are unique, possessing distinct masses and properties that differentiate them from atoms of other elements. This explains the diverse characteristics of different substances.
Significance: This postulate is crucial for distinguishing between different elements and understanding their unique behavior. It's the basis for the periodic table, which organizes elements based on their atomic number (number of protons) and resulting properties. Different elements react differently, form different compounds, and exhibit different physical characteristics.
Limitations: This postulate, while largely true, needs to be contextualized in terms of isotopes. While elements differ in their standard atomic mass, isotopes introduce a degree of variability. However, the overall properties of an element are defined by the average mass of its isotopes and their relative abundance, as reflected in the element's atomic weight on the periodic table.
Postulate 4: Atoms Combine in Simple, Whole-Number Ratios to Form Chemical Compounds
This postulate explains the composition of compounds. Dalton proposed that atoms combine in simple, whole-number ratios to create compounds. For example, water (H₂O) always contains two hydrogen atoms for every one oxygen atom, reflecting a 2:1 ratio. This principle underlies the law of definite proportions.
Significance: This postulate explains the law of definite proportions, which states that a given compound always contains the same elements in the same proportion by mass. It provides a quantitative framework for understanding compound formation and chemical formulas. This also enables the precise calculation of the amount of reactants needed for a reaction and the amount of products that will be produced.
Limitations: While true for many compounds, this postulate fails to account for the existence of non-stoichiometric compounds, where the ratio of elements is not a simple whole number. This is particularly relevant in solid-state chemistry, where crystal defects can lead to variations in elemental ratios. However, for most simple compounds, the postulate holds true.
Postulate 5: Atoms Are Rearranged but Not Created or Destroyed in Chemical Reactions
This postulate, also known as the law of conservation of mass, is a cornerstone of chemistry. Dalton stated that during chemical reactions, atoms are neither created nor destroyed; they are simply rearranged to form new combinations. The total mass remains constant throughout the reaction.
Significance: This postulate is vital for balancing chemical equations and for understanding the quantitative aspects of chemical transformations. It ensures that the number of atoms of each element remains the same on both sides of a balanced chemical equation. This conservation of mass simplifies quantitative calculations in chemistry, ensuring mass balance in reactions.
Limitations: While largely true for chemical reactions, this postulate does not hold for nuclear reactions. In nuclear reactions, atoms can be transformed, and mass can be converted into energy (as described by Einstein's famous equation, E=mc²). However, for typical chemical reactions at standard temperatures and pressures, the law of conservation of mass remains a fundamental principle.
Conclusion: The Enduring Legacy of Dalton's Atomic Theory
Despite some limitations, Dalton's atomic theory remains a monumental achievement in the history of science. It provided a fundamental framework for understanding the nature of matter, leading to significant advances in chemistry and related fields. While some postulates required modifications in light of later discoveries (like isotopes and subatomic particles), the core concepts of atoms as fundamental building blocks of matter and their combination in simple ratios remain cornerstone principles of modern chemistry. The theory's elegance and explanatory power continue to inspire scientific inquiry and provide a solid foundation for understanding the macroscopic world from a microscopic perspective. The theory's influence extends far beyond the basic concepts it introduced; it serves as a prime example of how a scientific model, despite its limitations, can be foundational to progress and further refinement in understanding the natural world. It laid the groundwork for future advancements in quantum mechanics and our current understanding of atomic structure, showcasing the iterative nature of scientific progress and the importance of building upon previous knowledge.
Latest Posts
Latest Posts
-
Current As A Function Of Time
Mar 18, 2025
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about 5 Postulates Of Dalton's Atomic Theory . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
