A Que Temperatura Se Congela El Agua En Fahrenheit
Muz Play
Mar 24, 2025 · 6 min read
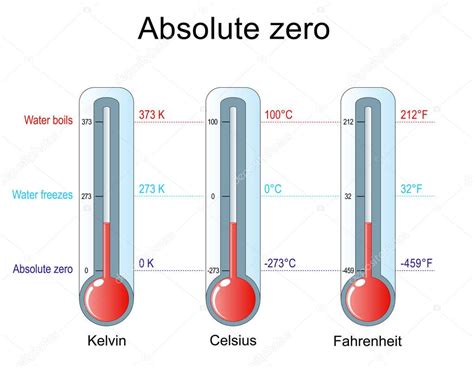
Table of Contents
At What Temperature Does Water Freeze in Fahrenheit?
Water freezing is a fundamental process in nature, impacting everything from weather patterns to the survival of aquatic life. Understanding the precise temperature at which this transition occurs is crucial in various fields, from science and engineering to everyday life. So, at what temperature does water freeze in Fahrenheit? The simple answer is 32°F. However, this seemingly straightforward answer opens up a fascinating exploration of the nuances surrounding water's freezing point.
Understanding the Freezing Point of Water
The freezing point of water, the temperature at which it transitions from a liquid to a solid (ice), is a critical physical property. While commonly stated as 32°F (or 0°C), this value is under specific standard conditions. Several factors can influence this point, making it a more complex phenomenon than initially perceived.
Standard Conditions and Variations
The accepted freezing point of 32°F is established under standard atmospheric pressure (one atmosphere, or 14.7 pounds per square inch). Variations in pressure can slightly affect the freezing point. Increased pressure slightly lowers the freezing point, while decreased pressure slightly raises it. This is why water can remain liquid at temperatures slightly below 32°F at high altitudes, where atmospheric pressure is lower. This effect is known as pressure-induced freezing point depression.
The Role of Impurities
Pure water freezes at 32°F. However, the presence of impurities, such as dissolved salts or other substances, can significantly alter the freezing point. This is the principle behind the use of antifreeze in car radiators. Antifreeze contains substances that lower the freezing point of water, preventing the coolant from freezing in cold weather. This phenomenon is known as freezing point depression. The more impurities present, the lower the freezing point will be. Seawater, for example, freezes at a temperature slightly below 32°F due to its salt content.
Supercooling: Water's Mysterious Delay
Sometimes, water can remain liquid even below its freezing point, a phenomenon known as supercooling. This occurs when the water lacks sufficient nucleation sites – tiny particles or imperfections that act as starting points for ice crystal formation. Without these sites, the water molecules may not have the necessary structure to begin forming ice crystals, even when the temperature drops below 32°F. However, once a nucleation site is introduced, the supercooled water will rapidly freeze. This can be seen in the instantaneous freezing of supercooled water when a small ice crystal or other particle is added.
The Importance of Nucleation Sites
Understanding the role of nucleation sites is essential in various applications. For example, in cloud formation, tiny particles in the atmosphere (aerosols) act as nucleation sites for ice crystal formation. Similarly, in freezing food, controlled nucleation is used to optimize the ice crystal formation process, minimizing damage to food cells and preserving quality.
Practical Applications of Water's Freezing Point
The knowledge of water's freezing point has countless practical applications across various fields.
Food Preservation
Freezing is a common method of food preservation, relying on the principle of lowering the temperature of food below its freezing point to inhibit microbial growth and slow down enzymatic reactions that cause spoilage. The specific freezing temperature, however, needs to be considered based on the food type and its water content to prevent damage to the food's texture and quality.
Weather Forecasting
Understanding the freezing point of water is crucial in weather forecasting. The formation of ice, snow, and frost are directly related to temperatures dropping below 32°F. Accurate predictions of freezing temperatures are vital for issuing warnings about potential hazards such as icy roads and severe winter weather conditions.
Construction and Engineering
Freezing and thawing cycles significantly affect the properties of construction materials. The expansion of water as it freezes can cause cracking and damage in concrete and other materials, making an understanding of water's freezing point critical for infrastructure design and maintenance. Engineers must take into account the potential for freezing and thawing to ensure the longevity and stability of structures.
Cryogenics and Refrigeration
The freezing point of water is fundamental to cryogenics, the study and application of very low temperatures. The process of freezing substances involves manipulating the freezing point by using various refrigerants and techniques. The cooling systems in refrigerators, freezers, and air conditioners all operate based on principles related to the freezing point of water and other refrigerants.
Medical Applications
Freezing is employed in several medical procedures, such as cryosurgery, which uses extremely low temperatures to destroy abnormal tissues. The precise control of temperature is crucial in these applications, requiring a deep understanding of freezing processes and the behavior of water under various conditions.
Beyond the Basics: Exploring Related Concepts
Understanding the freezing point of water extends beyond simply knowing the numerical value of 32°F. It opens up avenues to explore related concepts, fostering a deeper appreciation of the intricacies of this fundamental physical process.
Latent Heat of Fusion
When water freezes, it releases energy in the form of latent heat of fusion. This is the energy required to change the state of water from liquid to solid without a change in temperature. This released energy is important in various processes, such as the moderating effect of large bodies of water on climate.
Ice Crystal Formation
The formation of ice crystals is a complex process that involves the organization of water molecules into a hexagonal lattice structure. The shape and size of these crystals influence the properties of ice, such as its density and its ability to reflect light, creating diverse natural phenomena like snowflakes.
Density Anomaly of Water
Water exhibits a unique property: it is less dense as a solid (ice) than as a liquid. This anomalous behavior is crucial for aquatic life, as it ensures that ice floats on water, providing insulation for organisms living beneath the ice in cold climates.
Conclusion: The Significance of 32°F
The freezing point of water at 32°F is far more than just a number; it's a cornerstone of various scientific, engineering, and everyday applications. Understanding this fundamental property, along with the factors that can influence it, is vital in various fields, from food preservation and weather forecasting to construction and medicine. The seemingly simple fact of water freezing at 32°F under standard conditions opens up a vast and intricate world of scientific principles and practical applications, highlighting the importance of continued exploration and understanding of this essential natural process. From the formation of snowflakes to the design of robust infrastructure, the temperature of 32°F plays a pivotal role in shaping our world.
Latest Posts
Latest Posts
-
What Does A Negative Delta G Mean
Mar 26, 2025
-
What Is The Reactants Of Glycolysis
Mar 26, 2025
-
An Introduction To General Organic And Biological Chemistry
Mar 26, 2025
-
The Cutaneous Membrane Is Also Known As
Mar 26, 2025
-
Solve The Following System Of Equations Algebraically
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about A Que Temperatura Se Congela El Agua En Fahrenheit . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
