A Solution Contains Dissolved Substances Called
Muz Play
Mar 23, 2025 · 6 min read
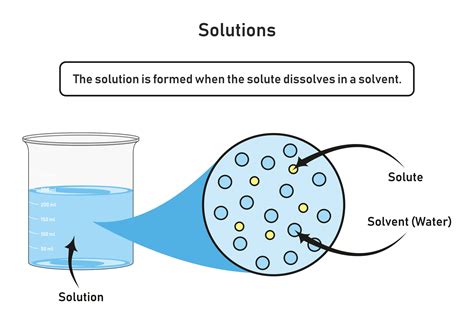
Table of Contents
A Solution Contains Dissolved Substances Called: Solutes – A Deep Dive into Solutions Chemistry
Solutions are ubiquitous in our daily lives, from the saltwater in the ocean to the electrolytes in our bodies. Understanding what constitutes a solution, and specifically, the dissolved substances within it, is fundamental to many scientific fields and everyday applications. This article delves into the chemistry of solutions, focusing on the dissolved substances called solutes, their properties, and their behavior within different solvents.
Understanding Solutions: A Mixture of Solutes and Solvents
A solution is a homogeneous mixture composed of two or more substances. Crucially, these substances are completely mixed at a molecular level, meaning you cannot visually distinguish the individual components. This homogeneity is key; a solution is uniform throughout. Unlike heterogeneous mixtures like sand and water, where distinct components remain separate, the components of a solution are inseparable by simple physical methods like filtration.
This mixture consists of two primary components:
-
Solvent: This is the substance that dissolves other substances. It's usually present in the larger amount. Water is a very common solvent, often referred to as the "universal solvent" due to its ability to dissolve a wide range of substances. Other solvents include ethanol, acetone, and benzene.
-
Solute: This is the substance that is dissolved in the solvent. It's typically present in a smaller amount than the solvent. Examples include salt (NaCl) when dissolved in water, sugar (sucrose) in tea, and carbon dioxide (CO2) in carbonated drinks. This is the focus of our exploration.
Properties of Solutes Affecting Solubility
The ability of a solute to dissolve in a solvent is called solubility. Several factors influence a solute's solubility:
-
Nature of the Solute and Solvent: The "like dissolves like" rule is a guiding principle. Polar solutes (those with uneven charge distribution, like sugar) tend to dissolve well in polar solvents (like water), while nonpolar solutes (like fats) dissolve better in nonpolar solvents (like oil). This is due to the interactions between the molecules; similar intermolecular forces lead to better solubility.
-
Temperature: The effect of temperature on solubility varies. For most solid solutes, increasing the temperature increases their solubility in a liquid solvent. However, the solubility of gases in liquids generally decreases with increasing temperature. This is because higher temperatures increase the kinetic energy of gas molecules, making them more likely to escape the liquid phase.
-
Pressure: Pressure primarily affects the solubility of gases in liquids. Increasing pressure increases the solubility of a gas. This is why carbonated drinks lose their fizz when opened; the pressure is released, and the dissolved carbon dioxide escapes.
Types of Solutes and Their Interactions with Solvents
Solutes can be categorized in several ways:
1. Based on Chemical Nature:
-
Ionic Solutes: These solutes are composed of ions (charged atoms or molecules). When dissolved in water, they dissociate into their constituent ions. For example, sodium chloride (NaCl) dissociates into Na⁺ and Cl⁻ ions. The interaction between these ions and the polar water molecules is crucial for their solubility. The slightly negative oxygen atoms in water molecules are attracted to the positive sodium ions, while the slightly positive hydrogen atoms are attracted to the negative chloride ions. This process is called hydration.
-
Molecular Solutes: These solutes consist of molecules, rather than ions. Examples include sugar (sucrose) and ethanol. Their solubility depends on the polarity of the molecule and its ability to form intermolecular forces (like hydrogen bonds or dipole-dipole interactions) with the solvent molecules.
-
Metallic Solutes: Certain metals can dissolve in liquid mercury (forming amalgams), although this is less common than ionic or molecular solute dissolution. The interaction involves metallic bonding between the solute and mercury atoms.
2. Based on their effect on solutions:
-
Electrolytes: These solutes produce ions when dissolved in a solvent, resulting in an electrically conductive solution. Ionic compounds are typically strong electrolytes, meaning they completely dissociate into ions. Weak electrolytes partially dissociate, while nonelectrolytes do not produce ions when dissolved.
-
Nonelectrolytes: These solutes do not produce ions when dissolved, and therefore do not conduct electricity. Examples include sugar and many organic molecules.
3. Based on Concentration:
The concentration of a solute describes the amount of solute present in a given amount of solution. This can be expressed in various ways, including:
-
Molarity (M): Moles of solute per liter of solution.
-
Molality (m): Moles of solute per kilogram of solvent.
-
Percent by mass (% w/w): Grams of solute per 100 grams of solution.
-
Percent by volume (% v/v): Milliliters of solute per 100 milliliters of solution.
-
Parts per million (ppm) and parts per billion (ppb): These are used for very dilute solutions.
The Role of Intermolecular Forces in Solution Formation
The process of dissolving a solute involves overcoming the intermolecular forces holding the solute particles together and creating new interactions between the solute and solvent particles. The strength of these interactions determines the solubility of the solute.
-
Solute-Solute Interactions: These forces hold the solute particles together in their solid or liquid state. For example, ionic compounds are held together by strong electrostatic attractions between oppositely charged ions. Molecular compounds are held together by weaker intermolecular forces like hydrogen bonding, dipole-dipole interactions, and London dispersion forces.
-
Solvent-Solvent Interactions: These forces hold the solvent molecules together. Similar to solute-solute interactions, they can be strong or weak depending on the nature of the solvent molecules.
-
Solute-Solvent Interactions: These are the forces that form between the solute and solvent particles once the solute is dissolved. The strength of these interactions determines the solubility of the solute. If the solute-solvent interactions are stronger than the solute-solute and solvent-solvent interactions, the solute will dissolve readily. This is why polar solutes dissolve well in polar solvents – strong dipole-dipole interactions and hydrogen bonds can form between them.
Applications of Solution Chemistry
Understanding solutions and their properties is crucial in numerous applications across various scientific disciplines and everyday life:
-
Medicine: Intravenous fluids, oral medications, and many other pharmaceuticals are solutions. The solubility of drugs is vital for their absorption and efficacy.
-
Biology: Biological systems are largely aqueous solutions. The proper balance of electrolytes and other solutes in bodily fluids is essential for maintaining health.
-
Environmental Science: Understanding the solubility of pollutants is crucial for assessing their environmental impact and developing remediation strategies.
-
Industrial Chemistry: Many industrial processes involve solutions. Chemical reactions often occur more efficiently in solution, and solutions are used in cleaning, coating, and many other applications.
-
Food Science: Many food products are solutions or suspensions, and the solubility of various components affects their taste, texture, and shelf life.
Conclusion: The Significance of Solutes in Solution Chemistry
The dissolved substances called solutes are integral to the nature and functionality of solutions. Their properties, interactions with the solvent, and concentrations profoundly influence the characteristics and applications of these ubiquitous mixtures. Understanding the factors affecting solubility and the diverse roles of solutes is essential across a wide range of scientific and technological fields, from medicine and biology to environmental science and industrial processes. The depth of knowledge surrounding solutes and solutions continues to expand, with ongoing research uncovering new insights and applications for this fundamental area of chemistry. Therefore, a thorough grasp of solution chemistry is vital for anyone seeking to understand the world around us.
Latest Posts
Latest Posts
-
What Are The Building Blocks Of Fats
Mar 24, 2025
-
Magnetic Field Of A Point Charge
Mar 24, 2025
-
Ejercicios Con Objeto Directo E Indirecto
Mar 24, 2025
-
Gases Have A Definite Shape And Volume
Mar 24, 2025
-
Find The Intersection Of The Line And The Plane
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about A Solution Contains Dissolved Substances Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
