Gases Have A Definite Shape And Volume
Muz Play
Mar 24, 2025 · 5 min read
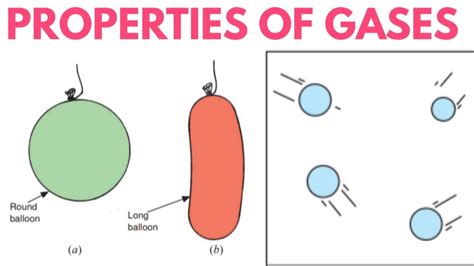
Table of Contents
Gases: Dispelling the Myth of Indefinite Shape and Volume
The statement "gases have a definite shape and volume" is fundamentally incorrect. Gases, unlike solids and liquids, are characterized by their lack of a fixed shape and volume. This is a cornerstone concept in chemistry and physics, stemming from the nature of intermolecular forces and the kinetic energy of gas particles. Understanding this crucial distinction is vital for comprehending various phenomena, from atmospheric pressure to the behavior of ideal gases. This article will delve deep into the properties of gases, exploring why they lack definite shape and volume, and examining the factors influencing their behavior.
The Kinetic Molecular Theory of Gases: The Foundation of Understanding
The behavior of gases is best explained using the kinetic molecular theory (KMT). This theory posits several key assumptions about the nature of gases:
- Gases are composed of tiny particles (atoms or molecules) that are in constant, random motion. This ceaseless movement is the primary reason why gases fill their containers completely.
- The volume of these particles is negligible compared to the total volume of the gas. This implies that the gas particles themselves occupy a minuscule fraction of the space the gas occupies.
- The attractive forces between gas particles are weak or negligible. This means that the particles are essentially independent of each other, unlike in liquids and solids where intermolecular forces significantly impact behavior.
- Collisions between gas particles and the container walls are elastic. This means that no kinetic energy is lost during collisions; the total kinetic energy of the system remains constant.
- The average kinetic energy of the gas particles is directly proportional to the absolute temperature (Kelvin). Higher temperatures mean faster-moving particles and thus increased pressure.
Why Gases Don't Have a Definite Shape
Imagine a balloon filled with air. The air molecules within the balloon are constantly moving in random directions at high speeds. They collide with each other and with the balloon's walls. Because the intermolecular forces between these air molecules are weak, they don't stick together or maintain any specific arrangement. Therefore, the gas expands to fill the entire volume of the container, adopting its shape completely. If you were to change the shape of the container (e.g., by squeezing the balloon), the gas would immediately conform to the new shape. This inherent adaptability to the container's form is why gases lack a definite shape.
The Role of Intermolecular Forces:
While the KMT assumes negligible intermolecular forces, it's important to note that real gases do experience weak attractive forces, particularly at lower temperatures and higher pressures. These forces, such as van der Waals forces, can slightly influence gas behavior, causing deviations from the ideal gas law. However, these forces are generally too weak to impose a definite shape on the gas.
Why Gases Don't Have a Definite Volume
The volume of a gas is not fixed; it's defined by the container it occupies. Unlike solids and liquids, where the particles are relatively close together, gas particles are widely dispersed. The vast empty space between gas molecules allows them to be easily compressed or expanded. If you decrease the volume of the container, the gas molecules will be forced closer together, resulting in a higher density and pressure. Conversely, if you increase the volume of the container, the gas will expand to fill the available space, reducing its density and pressure.
Compressibility and Expandability:
The compressibility and expandability of gases are direct consequences of the large intermolecular distances. This property is exploited in various applications, from pneumatic systems in vehicles to the storage of gases in compressed cylinders.
Real Gases vs. Ideal Gases: Bridging the Gap
The KMT describes an ideal gas, a theoretical construct that perfectly obeys all the assumptions of the KMT. However, real gases deviate from ideal behavior, especially at high pressures and low temperatures. At high pressures, the volume of the gas particles becomes significant relative to the total volume, and intermolecular forces become more prominent. At low temperatures, the kinetic energy of the particles is reduced, making the attractive forces more influential.
The van der Waals Equation:
The van der Waals equation is a modification of the ideal gas law that attempts to account for the non-ideal behavior of real gases. It incorporates correction factors to address the volume occupied by the gas particles and the attractive forces between them.
Applications and Everyday Examples
The properties of gases are fundamental to numerous applications and everyday phenomena:
- Weather patterns: Atmospheric pressure, wind, and temperature fluctuations are all governed by the behavior of gases in the atmosphere.
- Respiration: Gases like oxygen and carbon dioxide are crucial for respiration in living organisms.
- Combustion engines: Internal combustion engines rely on the expansion of gases produced during the combustion of fuel.
- Aerosols: Many everyday products, such as sprays and deodorants, utilize compressed gases to dispense their contents.
- Inflation: Inflatable objects, from balloons to tires, rely on the ability of gases to fill and expand within a container.
- Refrigeration and Air Conditioning: These systems utilize the properties of gases to transfer heat, maintaining comfortable temperatures.
- Diving and Underwater Activities: Understanding gas behavior under pressure is crucial for scuba diving and other underwater activities.
Conclusion: Understanding the Indefinite Nature of Gases
In conclusion, the statement that gases have a definite shape and volume is inaccurate. Gases are characterized by their lack of a fixed shape and volume. Their behavior is governed by the kinetic molecular theory, which highlights the constant, random motion of gas particles, their negligible volume relative to the container, and the weak intermolecular forces between them. Understanding the behavior of gases is crucial in various fields, from meteorology and chemistry to engineering and medicine. While ideal gas behavior provides a simplified model, it's essential to remember that real gases exhibit deviations from ideality, especially under extreme conditions. This understanding of gas properties allows for technological advancements and improved comprehension of numerous natural processes.
Latest Posts
Latest Posts
-
Advertising Goals Listed In An Advertising Plan Must Be
Mar 26, 2025
-
What Do Hypotheses Theories And Laws Have In Common
Mar 26, 2025
-
What Are The Products Of Neutralization
Mar 26, 2025
-
What Structure Is Most Important In Forming The Tetrads
Mar 26, 2025
-
What Color Is An Animal Cell
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Gases Have A Definite Shape And Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
