What Are The Products Of Neutralization
Muz Play
Mar 26, 2025 · 6 min read
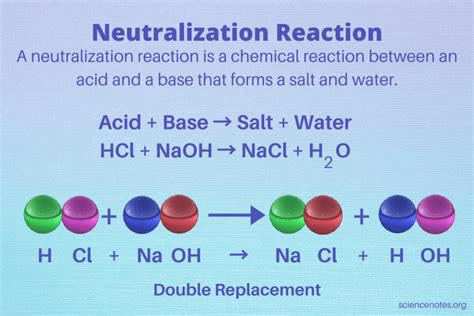
Table of Contents
- What Are The Products Of Neutralization
- Table of Contents
- What Are the Products of Neutralization? A Deep Dive into Acid-Base Reactions
- Understanding Neutralization Reactions: The Basics
- The Products: A Closer Look
- 1. Water (H₂O)
- 2. Salt
- Factors Influencing Neutralization Reactions
- 1. Strength of Acid and Base
- 2. Concentration of Acid and Base
- 3. Temperature
- 4. Stoichiometry
- Types of Neutralization Reactions and Their Products
- 1. Strong Acid-Strong Base Neutralization
- 2. Strong Acid-Weak Base Neutralization
- 3. Weak Acid-Strong Base Neutralization
- 4. Weak Acid-Weak Base Neutralization
- Applications of Neutralization Reactions
- 1. Industrial Processes
- 2. Environmental Applications
- 3. Biological Systems
- Conclusion: The Ubiquity of Neutralization
- Latest Posts
- Latest Posts
- Related Post
What Are the Products of Neutralization? A Deep Dive into Acid-Base Reactions
Neutralization reactions are fundamental chemical processes that underpin countless natural phenomena and industrial applications. Understanding the products of these reactions is crucial for anyone studying chemistry, environmental science, or related fields. This comprehensive guide explores the intricacies of neutralization, detailing the products formed, the factors influencing the reaction, and real-world examples of its significance.
Understanding Neutralization Reactions: The Basics
Neutralization, at its core, is a chemical reaction between an acid and a base. This reaction typically results in the formation of water and a salt. The driving force behind this reaction is the transfer of protons (H⁺ ions) from the acid to the base.
Let's break this down further:
- Acids: Substances that donate protons (H⁺ ions) when dissolved in water. Examples include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), and acetic acid (CH₃COOH).
- Bases: Substances that accept protons (H⁺ ions) when dissolved in water. Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), and ammonia (NH₃).
The general equation for a neutralization reaction is:
Acid + Base → Salt + Water
The Products: A Closer Look
The two primary products of a neutralization reaction are:
1. Water (H₂O)
The formation of water is a key characteristic of neutralization. The protons (H⁺) from the acid combine with hydroxide ions (OH⁻) from the base to form water molecules. This is an exothermic reaction, meaning it releases heat. The heat released can vary depending on the strength of the acid and base involved. Strong acid-strong base reactions release the most heat.
2. Salt
The "salt" formed in a neutralization reaction is not necessarily the common table salt (sodium chloride, NaCl). The term "salt" in chemistry refers to any ionic compound formed from the cation of the base and the anion of the acid. The properties of the salt formed are highly dependent on the specific acid and base used in the reaction.
Let's illustrate this with some examples:
- HCl (hydrochloric acid) + NaOH (sodium hydroxide) → NaCl (sodium chloride) + H₂O (water)
In this reaction, the sodium cation (Na⁺) from the base combines with the chloride anion (Cl⁻) from the acid to form sodium chloride, common table salt.
- H₂SO₄ (sulfuric acid) + 2KOH (potassium hydroxide) → K₂SO₄ (potassium sulfate) + 2H₂O (water)
Here, the potassium cation (K⁺) from the base combines with the sulfate anion (SO₄²⁻) from the acid to form potassium sulfate.
- CH₃COOH (acetic acid) + NH₃ (ammonia) → CH₃COONH₄ (ammonium acetate) + H₂O (water)
This reaction produces ammonium acetate, a salt commonly used as a buffer in biological systems.
Factors Influencing Neutralization Reactions
Several factors influence the outcome and characteristics of a neutralization reaction:
1. Strength of Acid and Base
The strength of the acid and base involved significantly impacts the reaction's heat release and the pH of the resulting solution. Strong acids and bases completely dissociate in water, leading to a more vigorous reaction and a neutral pH (pH 7) if stoichiometrically equivalent amounts are used. Weak acids and bases partially dissociate, resulting in a less vigorous reaction and a pH that may not be exactly neutral.
2. Concentration of Acid and Base
The concentration of the acid and base directly affects the reaction rate. Higher concentrations lead to faster reactions. The final pH of the solution also depends on the relative concentrations of the acid and base.
3. Temperature
Temperature influences the rate of reaction. Increasing the temperature generally speeds up the reaction rate, as it increases the kinetic energy of the reacting molecules.
4. Stoichiometry
The stoichiometry of the reaction—the relative amounts of acid and base—determines whether the resulting solution will be acidic, basic, or neutral. If stoichiometrically equivalent amounts of acid and base are used, the resulting solution will be neutral. An excess of acid will result in an acidic solution, while an excess of base will result in a basic solution.
Types of Neutralization Reactions and Their Products
Neutralization reactions can be categorized based on the type of acid and base involved:
1. Strong Acid-Strong Base Neutralization
These reactions produce a neutral solution (pH 7) when stoichiometrically equivalent amounts are mixed. The salt formed is usually a neutral salt, meaning it does not significantly affect the pH of the solution.
2. Strong Acid-Weak Base Neutralization
These reactions produce a slightly acidic solution (pH < 7) even when stoichiometrically equivalent amounts are used. The salt formed is often slightly acidic due to hydrolysis.
3. Weak Acid-Strong Base Neutralization
These reactions produce a slightly basic solution (pH > 7) even when stoichiometrically equivalent amounts are used. The salt formed is often slightly basic due to hydrolysis.
4. Weak Acid-Weak Base Neutralization
These reactions are more complex and the pH of the resulting solution depends on the relative strengths of the acid and base involved. Predicting the exact pH requires considering the equilibrium constants of both the acid and the base.
Applications of Neutralization Reactions
Neutralization reactions have widespread applications across various fields:
1. Industrial Processes
Neutralization is used extensively in various industrial processes, including:
- Wastewater treatment: Neutralization is crucial in treating industrial wastewater to adjust its pH to acceptable levels before discharge.
- Chemical synthesis: Many chemical syntheses involve neutralization reactions to prepare specific salts or to control the pH of the reaction mixture.
- Food industry: Neutralization is used in food processing to adjust the pH of food products and to prevent spoilage.
2. Environmental Applications
Neutralization plays a significant role in:
- Acid rain mitigation: Liming, the addition of calcium carbonate (CaCO₃) to acidic soils and lakes, is a common method for neutralizing the effects of acid rain.
- Soil remediation: Neutralization is used to treat contaminated soils by adjusting the pH to levels suitable for plant growth.
3. Biological Systems
Neutralization is essential for maintaining the pH balance in biological systems:
- Blood buffering: The blood buffering system relies on neutralization reactions to maintain a stable pH, crucial for proper physiological function.
- Digestion: The digestive system uses neutralization reactions to maintain the optimal pH for enzyme activity.
Conclusion: The Ubiquity of Neutralization
Neutralization reactions are fundamental chemical processes with far-reaching implications. Understanding the products of these reactions – water and a salt – is key to comprehending their significance in various contexts, from industrial processes and environmental remediation to the maintenance of life itself. The factors influencing these reactions, such as the strength and concentration of the reactants and the temperature, must be considered for a complete understanding of their behavior. The ability to predict the pH of the resulting solution and the properties of the salt formed are crucial skills in chemistry and related fields. The diverse applications of neutralization highlight its importance in a wide array of fields, underlining its status as a cornerstone of chemistry and its contributions to our daily lives.
Latest Posts
Latest Posts
-
Which Of The Following Is An Organic Molecule
Mar 27, 2025
-
Magnetic Field For A Circular Loop
Mar 27, 2025
-
What Is The Most Challenging Issue Facing Genome Sequencing
Mar 27, 2025
-
Isotopes Have A Different Number Of
Mar 27, 2025
-
What Are Blocks In The Periodic Table
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about What Are The Products Of Neutralization . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
