What Are Blocks In The Periodic Table
Muz Play
Mar 27, 2025 · 6 min read
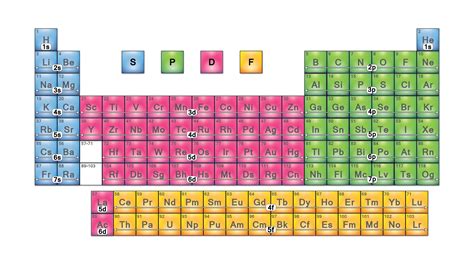
Table of Contents
What Are Blocks in the Periodic Table? A Deep Dive into s, p, d, and f Blocks
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. While rows (periods) represent increasing energy levels and columns (groups) reflect similar valence electron configurations, the table's structure is further refined by its division into blocks. These blocks – s, p, d, and f – provide crucial insights into the electronic structure and, consequently, the chemical behavior of elements. Understanding these blocks is key to unlocking a deeper comprehension of the periodic table's organization and predictive power.
Understanding Electron Configuration and its Relation to Blocks
Before diving into the specifics of each block, it's essential to grasp the concept of electron configuration. An element's electron configuration describes how electrons are distributed among different energy levels (shells) and sublevels (subshells) within an atom. These sublevels are designated by the letters s, p, d, and f, directly correlating to the blocks in the periodic table.
Each sublevel can accommodate a specific number of electrons:
- s sublevel: Holds a maximum of 2 electrons.
- p sublevel: Holds a maximum of 6 electrons.
- d sublevel: Holds a maximum of 10 electrons.
- f sublevel: Holds a maximum of 14 electrons.
The filling of these sublevels follows the Aufbau principle (electrons fill lower energy levels first) and Hund's rule (electrons individually occupy orbitals within a sublevel before pairing up). This systematic filling dictates the placement of elements within the specific blocks.
The s-Block: Alkali Metals and Alkaline Earth Metals
The s-block elements occupy the first two groups of the periodic table. These elements are characterized by their valence electrons residing in the s sublevel.
Group 1: Alkali Metals (excluding Hydrogen)
Alkali metals (Li, Na, K, Rb, Cs, Fr) are highly reactive metals with one valence electron in their outermost s orbital (ns¹ configuration). This single electron is easily lost, resulting in the formation of +1 ions. Their reactivity increases down the group due to the increasing atomic size and decreasing ionization energy. They are soft, silvery-white metals, with low melting and boiling points. They readily react with water to produce hydrogen gas and alkaline solutions.
Group 2: Alkaline Earth Metals
Alkaline earth metals (Be, Mg, Ca, Sr, Ba, Ra) have two valence electrons in their outermost s orbital (ns² configuration). They are also reactive metals, though less so than alkali metals, forming +2 ions. They exhibit higher melting and boiling points than alkali metals due to stronger metallic bonding. Their reactivity also increases down the group, although less dramatically than alkali metals.
The p-Block: A Diverse Range of Elements
The p-block elements occupy groups 13 to 18 (excluding helium). These elements are characterized by their valence electrons filling the p sublevel. This block encompasses a remarkably diverse range of elements, including nonmetals, metalloids (semi-metals), and some metals.
Group 13: Boron Group
Elements in this group (B, Al, Ga, In, Tl) have three valence electrons (ns²np¹ configuration). Boron is a metalloid, while the others are metals exhibiting a variety of oxidation states, although +3 is the most common.
Group 14: Carbon Group
The carbon group (C, Si, Ge, Sn, Pb) exhibits four valence electrons (ns²np² configuration). Carbon is a nonmetal crucial for organic chemistry, while silicon and germanium are metalloids with significant applications in semiconductors. Tin and lead are metals.
Group 15: Nitrogen Group (Pnictogens)
The nitrogen group (N, P, As, Sb, Bi) possesses five valence electrons (ns²np³ configuration). Nitrogen is a nonmetal forming various oxides and is essential to living organisms. Phosphorus, arsenic, and antimony are also nonmetals, although antimony can exhibit metallic properties. Bismuth is a metal.
Group 16: Chalcogens
The chalcogens (O, S, Se, Te, Po) have six valence electrons (ns²np⁴ configuration). Oxygen is crucial for respiration and is a highly reactive nonmetal. Sulfur, selenium, and tellurium are nonmetals with increasingly metallic character down the group. Polonium is a radioactive metal.
Group 17: Halogens
Halogens (F, Cl, Br, I, At) are highly reactive nonmetals with seven valence electrons (ns²np⁵ configuration). They readily gain one electron to form -1 ions, and their reactivity decreases down the group. Their properties change significantly from gaseous fluorine to solid astatine.
Group 18: Noble Gases
Noble gases (He, Ne, Ar, Kr, Xe, Rn) are inert gases with a full outermost shell (ns²np⁶ configuration except for helium, which has a full 1s² shell). Their stability stems from their completed electron configuration, rendering them largely unreactive.
The d-Block: Transition Metals
The d-block elements, located in the middle of the periodic table, are characterized by the filling of the d sublevel. These elements are primarily transition metals, known for their variable oxidation states, catalytic activity, and often colorful compounds.
The d-block elements exhibit a wide range of properties. Their variable oxidation states are a consequence of the relatively close energies of the (n-1)d and ns orbitals. This allows for the participation of both s and d electrons in bonding, leading to multiple oxidation states. Many d-block elements act as catalysts in various chemical reactions, and their compounds often display vibrant colors due to the presence of partially filled d orbitals.
The f-Block: Inner Transition Metals (Lanthanides and Actinides)
The f-block elements, placed separately at the bottom of the periodic table, represent the filling of the f sublevel. These elements are further divided into two series:
Lanthanides
The lanthanides (Ce to Lu) are characterized by the filling of the 4f sublevel. They are all silvery-white metals with very similar chemical properties due to the shielding effect of the 5s and 5p electrons.
Actinides
The actinides (Th to Lr) are characterized by the filling of the 5f sublevel. Most actinides are radioactive, and many are synthetically produced. Their properties are less uniform compared to lanthanides due to the relativistic effects influencing the 5f electrons.
The Significance of Block Classification
The classification of elements into blocks is not merely a structural feature of the periodic table; it provides invaluable insights into their properties and behavior. Understanding the block to which an element belongs instantly allows for predictions about its:
- Oxidation states: The number of valence electrons directly influences the possible oxidation states an element can adopt.
- Reactivity: The completeness of the valence shell significantly impacts an element's reactivity. Elements with nearly full or empty valence shells tend to be more reactive.
- Bonding behavior: The type of orbitals involved in bonding (s, p, d, or f) dictates the types of bonds an element can form.
- Physical properties: The electron configuration strongly correlates with various physical properties like melting point, boiling point, density, and conductivity.
Conclusion: Blocks as a Foundation for Chemical Understanding
The s, p, d, and f blocks of the periodic table represent a fundamental organizational framework based on electron configurations. This organization isn't arbitrary; it is deeply rooted in the underlying principles of atomic structure and quantum mechanics. By understanding the characteristics of each block, we can predict and explain the remarkable diversity of chemical behavior exhibited by elements. This block-based classification acts as a powerful tool for chemists and students alike, providing a foundation for a more profound understanding of the periodic table and the fascinating world of chemistry. The periodic table, therefore, is not just a static arrangement of elements, but a dynamic representation of the intricate relationships governing their properties and interactions. This knowledge enables us to predict and manipulate chemical reactions, design new materials, and understand the fundamental building blocks of our universe.
Latest Posts
Latest Posts
-
What Type Of Ion Will Calcium Form
Mar 30, 2025
-
The Atomic Mass Number Is Equal To
Mar 30, 2025
-
Where Does Dna Replication Take Place In A Eukaryotic Cell
Mar 30, 2025
-
Find Standard Matrix Of Linear Transformation
Mar 30, 2025
-
Is Delta H Products Minus Reactants
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Are Blocks In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
