The Atomic Mass Number Is Equal To
Muz Play
Mar 30, 2025 · 5 min read
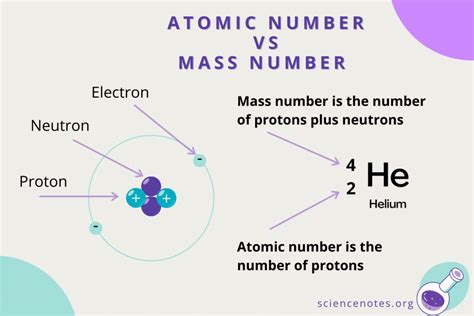
Table of Contents
The Atomic Mass Number is Equal To: A Deep Dive into Atomic Structure and Isotopes
Understanding the atomic mass number is fundamental to grasping the intricacies of chemistry and physics. This seemingly simple number holds a wealth of information about an atom, revealing its composition and behavior. This comprehensive guide will delve into the meaning of atomic mass number, explore its relationship with other atomic properties, discuss isotopes and their impact on atomic mass, and finally, illustrate its significance in various scientific applications.
What is Atomic Mass Number?
The atomic mass number (also known as the mass number) is the total number of protons and neutrons found in the nucleus of an atom. It's represented by the letter 'A' and is an integer value. Crucially, it does not include the number of electrons, as their mass is negligible compared to that of protons and neutrons.
In simpler terms: Imagine an atom as a tiny solar system. The nucleus, at the center, is composed of protons (positively charged particles) and neutrons (neutral particles). The electrons orbit the nucleus much like planets orbiting the sun. The atomic mass number simply counts all the particles in the nucleus.
For example, consider a carbon atom. A carbon atom typically has 6 protons and 6 neutrons. Therefore, its atomic mass number (A) is 6 + 6 = 12. We represent this as ¹²C, where the superscript 12 is the mass number.
The Relationship Between Atomic Mass Number, Atomic Number, and Number of Neutrons
Three key numbers define the composition of an atom:
- Atomic Number (Z): This represents the number of protons in the nucleus. It uniquely identifies an element. For example, all carbon atoms have an atomic number of 6.
- Atomic Mass Number (A): The total number of protons and neutrons.
- Number of Neutrons (N): This is simply the difference between the atomic mass number and the atomic number: N = A - Z
This relationship is crucial for understanding the structure of an atom. Knowing any two of these numbers allows us to calculate the third.
Example:
Let's take the isotope ¹⁴C (Carbon-14).
- Its atomic mass number (A) is 14.
- Its atomic number (Z), being carbon, is 6.
- Therefore, the number of neutrons (N) is 14 - 6 = 8.
Isotopes and Their Impact on Atomic Mass Number
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This means they have the same atomic number (Z) but different atomic mass numbers (A).
Because isotopes have varying numbers of neutrons, they have different atomic masses. While the number of protons determines the element's identity, the number of neutrons influences its stability and mass. Some isotopes are stable, while others are radioactive, undergoing decay to become more stable.
Examples of Isotopes:
- Carbon-12 (¹²C): 6 protons, 6 neutrons. This is the most abundant and stable isotope of carbon.
- Carbon-13 (¹³C): 6 protons, 7 neutrons. A stable isotope of carbon.
- Carbon-14 (¹⁴C): 6 protons, 8 neutrons. A radioactive isotope of carbon, used in radiocarbon dating.
The atomic mass number differs for each isotope. While ¹²C has a mass number of 12, ¹⁴C has a mass number of 14.
Atomic Mass vs. Atomic Mass Number: A Crucial Distinction
It's essential to distinguish between atomic mass number and atomic mass.
- Atomic Mass Number (A): A whole number representing the sum of protons and neutrons in an atom's nucleus.
- Atomic Mass (or Atomic Weight): The average mass of all the isotopes of an element, weighted by their abundance in nature. This is usually not a whole number because it considers the different isotopes and their relative abundances.
For instance, the atomic mass of carbon is approximately 12.011 amu (atomic mass units), not exactly 12, because it's a weighted average of the masses of ¹²C, ¹³C, and other carbon isotopes.
Significance of Atomic Mass Number in Various Scientific Fields
The atomic mass number plays a pivotal role in numerous scientific disciplines:
1. Nuclear Chemistry and Physics:
Understanding the atomic mass number is crucial for predicting nuclear reactions, such as nuclear fission and fusion. The stability of an isotope is directly related to its neutron-to-proton ratio, which is calculated using the atomic mass number and atomic number. Radioactive decay processes, crucial in nuclear medicine and energy production, are also directly influenced by the atomic mass number.
2. Analytical Chemistry:
In mass spectrometry, the atomic mass number is used to identify and quantify different isotopes of an element in a sample. This technique has broad applications in various fields, including environmental monitoring, forensic science, and medical diagnostics.
3. Astrophysics and Cosmology:
The abundance of isotopes in stars and other celestial objects provides insights into the nucleosynthesis processes that formed these objects. The atomic mass number is essential for understanding these processes and tracing the evolution of the universe.
4. Geochemistry and Geology:
Radioactive isotopes with known half-lives and atomic mass numbers are used in radiometric dating techniques to determine the age of rocks, minerals, and fossils. This is crucial for understanding geological processes and the history of the Earth.
5. Medicine:
Radioactive isotopes, identified by their atomic mass numbers, are used in medical imaging techniques such as PET (Positron Emission Tomography) and SPECT (Single-Photon Emission Computed Tomography) to diagnose and monitor various diseases. Radioactive isotopes are also employed in radiotherapy to treat certain types of cancer.
Calculating Atomic Mass from Isotopic Abundances
As mentioned earlier, the atomic mass of an element is a weighted average of the masses of its isotopes. Here's how to calculate it:
Formula: Atomic Mass = (fractional abundance of isotope 1 × mass of isotope 1) + (fractional abundance of isotope 2 × mass of isotope 2) + ...
Example: Let's calculate the atomic mass of chlorine, which has two main isotopes: ³⁵Cl (75.77% abundance) and ³⁷Cl (24.23% abundance).
Atomic Mass of Chlorine = (0.7577 × 35 amu) + (0.2423 × 37 amu) ≈ 35.45 amu
This calculation demonstrates how the atomic mass reflects the relative abundances of different isotopes.
Conclusion: The Importance of a Simple Number
The atomic mass number, though seemingly simple, is a powerful tool for understanding the fundamental building blocks of matter. Its applications span a wide range of scientific fields, from nuclear physics to medical diagnostics. By understanding its relationship to atomic number, isotopes, and atomic mass, we gain deeper insights into the structure, properties, and behavior of atoms and the elements they compose. This knowledge underpins many advancements in science and technology, constantly shaping our understanding of the universe around us.
Latest Posts
Latest Posts
-
Integration By Parts How To Choose U And Dv
Apr 01, 2025
-
Protein Synthesis Takes Place In The
Apr 01, 2025
-
Microscopic Anatomy Of A Muscle Fiber
Apr 01, 2025
-
What Is The General Equation For Cellular Respiration
Apr 01, 2025
-
Are Influence Lines In The Fe Exam
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Atomic Mass Number Is Equal To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
