Isotopes Have A Different Number Of
Muz Play
Mar 27, 2025 · 7 min read
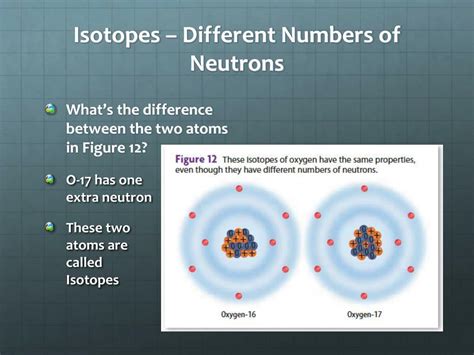
Table of Contents
Isotopes: Atoms with a Different Number of Neutrons
Isotopes are variations of a chemical element that have the same number of protons but differ in the number of neutrons within their atomic nuclei. This seemingly small difference has significant consequences, impacting the atom's mass, stability, and behavior in various chemical and physical processes. Understanding isotopes is crucial across numerous scientific fields, from nuclear physics and chemistry to medicine and environmental science. This comprehensive article delves into the fascinating world of isotopes, exploring their properties, applications, and implications.
Understanding Atomic Structure and Isotopes
Before diving into the specifics of isotopes, it's essential to review the basic structure of an atom. An atom consists of a central nucleus containing positively charged protons and neutral neutrons, orbited by negatively charged electrons. The number of protons, also known as the atomic number, defines the element. For example, all atoms with one proton are hydrogen, all atoms with six protons are carbon, and so on.
The mass number, however, represents the total number of protons and neutrons in the nucleus. This is where isotopes come into play. Isotopes of a given element possess the same number of protons but differ in their neutron count. This difference in neutron number directly influences the atom's mass, leading to variations in isotopic mass. Because the number of protons remains constant, the chemical properties of isotopes of the same element are largely similar. However, their physical properties, especially mass-dependent properties, can differ significantly.
Notation and Representation of Isotopes
Isotopes are commonly represented using a specific notation. The notation typically includes the element's symbol, its mass number (protons + neutrons) as a superscript, and its atomic number (number of protons) as a subscript. For example:
- ¹²C: This represents the most common isotope of carbon, with 6 protons and 6 neutrons (mass number 12).
- ¹⁴C: This is a radioactive isotope of carbon, with 6 protons and 8 neutrons (mass number 14).
The subscript atomic number is often omitted because the element symbol already uniquely identifies the number of protons. Therefore, you might frequently see isotopes represented simply as ¹²C and ¹⁴C.
Types of Isotopes: Stable and Radioactive
Isotopes can be broadly classified into two categories: stable and radioactive (also known as unstable).
Stable Isotopes
Stable isotopes are those that do not undergo radioactive decay. Their nuclei are stable and do not spontaneously transform into other nuclides (different atomic forms). The majority of naturally occurring isotopes are stable. The stability of an isotope is determined by the balance between the strong nuclear force (holding protons and neutrons together) and the electromagnetic force (repelling protons). A stable nucleus has a favorable neutron-to-proton ratio.
Radioactive Isotopes (Radioisotopes)
Radioactive isotopes, on the other hand, have unstable nuclei and undergo radioactive decay. This decay involves the emission of particles or energy, transforming the nucleus into a different nuclide. This process continues until a stable nucleus is reached. Radioactive decay can occur through various mechanisms, including alpha decay, beta decay, and gamma decay. Each type of decay involves the emission of different particles and changes the mass number and/or atomic number of the nucleus. The rate of radioactive decay is characterized by a half-life, representing the time it takes for half of the radioactive atoms in a sample to decay.
Applications of Isotopes
The unique properties of isotopes make them invaluable tools across diverse scientific and technological fields:
1. Medical Applications:
- Radioactive tracers: Radioisotopes like ¹³¹I (iodine-131) are used in medical imaging techniques such as PET (positron emission tomography) and SPECT (single-photon emission computed tomography) to diagnose and monitor various diseases. They help visualize organs and tissues, detecting abnormalities like tumors or infections.
- Radiotherapy: Radioisotopes such as cobalt-60 (⁶⁰Co) and cesium-137 (¹³⁷Cs) emit ionizing radiation used in radiotherapy to kill cancer cells.
- Radiopharmaceuticals: Radioactive isotopes incorporated into pharmaceutical compounds can target specific cells or tissues, delivering radiation to diseased areas while minimizing damage to healthy tissues.
2. Industrial Applications:
- Dating techniques: Radiocarbon dating using ¹⁴C is a widely employed method in archaeology and geology to determine the age of organic materials. The decay rate of ¹⁴C allows scientists to estimate the time elapsed since the organism's death.
- Industrial tracers: Isotopes are used as tracers to track the movement of materials in industrial processes, helping optimize efficiency and identify potential problems.
- Nuclear power generation: Nuclear power plants utilize the energy released from the controlled nuclear fission of isotopes like uranium-235 (²³⁵U).
3. Environmental Applications:
- Environmental monitoring: Isotopes are used to study environmental processes, such as water flow patterns, nutrient cycling, and pollutant dispersal. For example, the ratio of different oxygen isotopes in water samples can reveal information about the source and history of the water.
- Climate change research: The isotopic composition of ice cores and tree rings provides crucial data about past climate conditions, allowing scientists to reconstruct historical climate changes and study the effects of greenhouse gases.
4. Scientific Research:
- Nuclear physics research: Studying the properties of various isotopes helps scientists understand the fundamental forces governing the nucleus and the nature of nuclear reactions.
- Chemical analysis: Isotope ratio mass spectrometry (IRMS) is used in various fields for precise measurement of isotopic ratios, providing crucial information in various analytical applications.
- Geological studies: Isotopes are used to determine the age of rocks and minerals, helping geologists reconstruct the Earth's history and understand geological processes.
Isotopic Abundance and Average Atomic Mass
Naturally occurring elements exist as a mixture of different isotopes. The relative abundance of each isotope is typically constant, making it possible to calculate the average atomic mass of an element. The average atomic mass, often used in chemical calculations, is a weighted average of the masses of all naturally occurring isotopes of an element, considering their relative abundances.
For example, carbon exists primarily as two isotopes, ¹²C (98.93%) and ¹³C (1.07%). The average atomic mass of carbon is calculated as a weighted average of these two isotopes' masses and their relative abundances, resulting in approximately 12.01 atomic mass units (amu).
Isotope Effects
Despite having the same number of protons, isotopes can exhibit different physical and chemical properties due to their differing masses. These differences are known as isotope effects. These effects are more pronounced for lighter elements, where the relative mass difference between isotopes is larger.
Kinetic Isotope Effects
Kinetic isotope effects refer to the influence of isotopic substitution on the rates of chemical reactions. Heavier isotopes tend to react more slowly than lighter isotopes because of their lower vibrational frequencies. This effect is utilized in various chemical and biochemical studies.
Equilibrium Isotope Effects
Equilibrium isotope effects are observed in chemical equilibria where isotopic substitution affects the relative abundance of different isotopic species. Heavier isotopes tend to concentrate in molecules with stronger bonds. This effect finds applications in geochemical and environmental studies.
Separating Isotopes
Separating isotopes from each other is a challenging task because isotopes of the same element are chemically identical. Various techniques are used to achieve isotopic separation, each exploiting subtle differences in the physical properties of isotopes. These methods include:
- Gaseous diffusion: This method exploits the slightly different diffusion rates of gaseous isotopes.
- Centrifugation: This method uses high-speed centrifuges to separate isotopes based on their mass differences.
- Laser isotope separation: Lasers are used to selectively excite and ionize specific isotopes, allowing their separation.
- Electromagnetic separation: This method uses strong magnetic fields to separate ions of different isotopes based on their mass-to-charge ratio.
Conclusion
Isotopes represent variations of elements with the same number of protons but differing neutron counts. Their unique properties – particularly their different masses and radioactive decay patterns – have revolutionized numerous scientific and technological fields. From medical diagnosis and treatment to environmental monitoring and industrial processes, isotopes continue to play a vital role in advancing our understanding of the world around us. The continued research and development in isotope separation techniques and applications promise even further impactful discoveries and innovations in the years to come. The seemingly small difference in neutron number has profoundly significant consequences, highlighting the intricate and fascinating nature of atomic structure and its implications across diverse scientific disciplines.
Latest Posts
Latest Posts
-
Derivative Of Inverse Trig Functions Proof
Mar 30, 2025
-
The Carbon Atoms In Saturated Hydrocarbons
Mar 30, 2025
-
Formula For Area Of Cross Section
Mar 30, 2025
-
What Type Of Ion Will Calcium Form
Mar 30, 2025
-
The Atomic Mass Number Is Equal To
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Isotopes Have A Different Number Of . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
