The Carbon Atoms In Saturated Hydrocarbons
Muz Play
Mar 30, 2025 · 6 min read
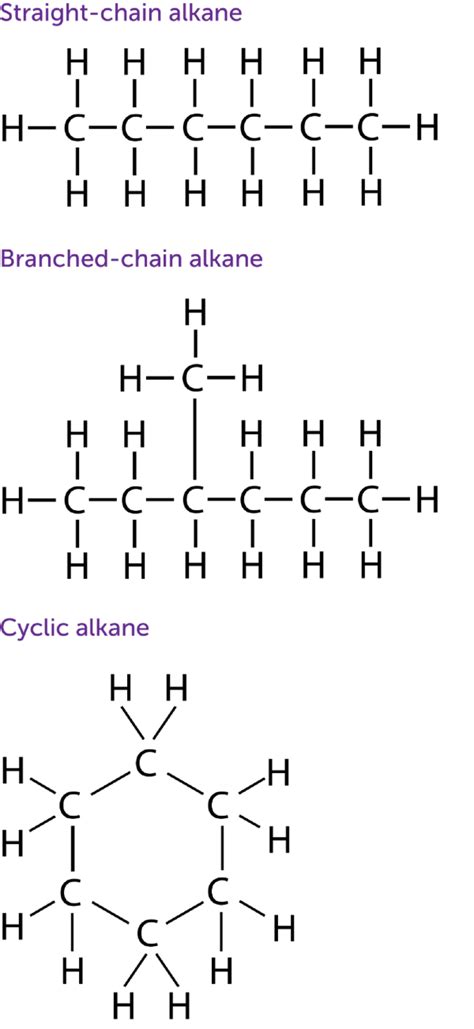
Table of Contents
Delving Deep into the Carbon Atoms in Saturated Hydrocarbons: A Comprehensive Guide
Saturated hydrocarbons, also known as alkanes, form the bedrock of organic chemistry. Their simple structure, characterized by single carbon-carbon bonds, belies a rich chemistry and significant industrial importance. Understanding the behavior and properties of the carbon atoms within these molecules is key to grasping their reactivity and applications. This comprehensive guide will explore the intricacies of carbon atoms in saturated hydrocarbons, covering their bonding, structure, properties, and applications.
The Fundamental Building Block: Carbon's Tetrahedral Geometry
At the heart of every saturated hydrocarbon lies the carbon atom. Carbon, with its four valence electrons, exhibits a remarkable ability to form four strong covalent bonds. In saturated hydrocarbons, these bonds are exclusively single bonds, resulting in a tetrahedral geometry around each carbon atom. This means that the four bonds are directed towards the corners of a tetrahedron, with bond angles of approximately 109.5 degrees. This specific geometry significantly influences the molecule's overall shape and properties.
Implications of Tetrahedral Geometry:
-
Shape and Conformation: The tetrahedral arrangement allows for free rotation around the single carbon-carbon bonds. This leads to a variety of possible conformations, or three-dimensional arrangements, for the molecule. Different conformations have slightly different energies, with some being more stable than others. This conformational flexibility is a crucial aspect of the behavior of saturated hydrocarbons.
-
Packing Efficiency: The tetrahedral geometry affects how efficiently saturated hydrocarbon molecules pack together in the solid and liquid states. This impacts properties like melting point and boiling point. Straight-chain alkanes tend to pack more efficiently than branched-chain alkanes, resulting in higher melting and boiling points for the straight-chain isomers.
-
Reactivity: The strong, single carbon-carbon bonds in saturated hydrocarbons are relatively unreactive compared to other types of carbon-carbon bonds (like double or triple bonds). This relative inertness accounts for their stability and makes them suitable for various applications where chemical stability is crucial. However, they can undergo reactions like combustion and halogenation under specific conditions.
Exploring the Carbon Chain: Straight-Chain vs. Branched-Chain Alkanes
Saturated hydrocarbons can exist as straight-chain alkanes or branched-chain alkanes. The arrangement of the carbon atoms significantly influences the physical and chemical properties of the molecule.
Straight-Chain Alkanes:
Straight-chain alkanes have a linear arrangement of carbon atoms. They are characterized by a relatively high degree of symmetry, which affects their physical properties. As the number of carbon atoms increases, the strength of the London Dispersion Forces (LDFs) between molecules increases, leading to higher melting and boiling points.
Branched-Chain Alkanes:
Branched-chain alkanes possess carbon atoms that branch off from the main chain. The presence of branches disrupts the linear arrangement and reduces the efficiency of packing. This leads to lower melting and boiling points compared to their straight-chain isomers with the same number of carbon atoms. Branched alkanes also exhibit lower reactivity due to steric hindrance caused by the bulky branches.
Isomerism in Alkanes:
The possibility of branched-chain alkanes introduces the concept of isomerism. Isomers are molecules with the same molecular formula but different structural formulas. For example, butane (C₄H₁₀) exists as both a straight-chain isomer (n-butane) and a branched-chain isomer (isobutane or methylpropane). These isomers have different physical and chemical properties.
The Influence of Carbon Atom Number: Trends in Properties
The number of carbon atoms in a saturated hydrocarbon significantly influences its physical properties. As the carbon chain length increases:
-
Boiling Point and Melting Point Increase: Larger molecules experience stronger London Dispersion Forces (LDFs) due to the increased surface area and number of electrons. These stronger intermolecular forces require more energy to overcome, resulting in higher boiling and melting points.
-
Viscosity Increases: Longer chains lead to increased entanglement and intermolecular interactions, resulting in higher viscosity (resistance to flow).
-
Solubility in Water Decreases: The nonpolar nature of alkanes makes them largely immiscible with water, a polar solvent. As the carbon chain lengthens, the hydrophobic character becomes more pronounced, further reducing solubility.
-
Flammability Remains Relatively Constant: While the amount of energy released during combustion increases with chain length, the flammability itself doesn't drastically change. All alkanes are flammable.
Chemical Reactions of Saturated Hydrocarbons: The Role of Carbon Atoms
Despite their relative inertness, saturated hydrocarbons do undergo specific chemical reactions, primarily involving the carbon-hydrogen bonds or, less frequently, the carbon-carbon bonds.
Combustion:
The most common reaction of saturated hydrocarbons is combustion, which involves the reaction with oxygen to produce carbon dioxide, water, and heat. This reaction is highly exothermic and is the basis for the use of alkanes as fuels. The complete combustion of alkanes yields carbon dioxide and water, whereas incomplete combustion can produce carbon monoxide and soot.
Halogenation:
Alkanes can react with halogens (fluorine, chlorine, bromine, iodine) in a process called halogenation. This reaction involves the substitution of one or more hydrogen atoms with halogen atoms. The reaction is usually initiated by UV light or heat and proceeds via a free radical mechanism. The reactivity of halogens decreases down the group (F₂ > Cl₂ > Br₂ > I₂).
Cracking:
Cracking is a process used in the petroleum industry to break down long-chain alkanes into smaller, more useful molecules. This process involves heating the alkanes in the absence of air to break the carbon-carbon bonds. Cracking produces a mixture of shorter-chain alkanes and alkenes, which are valuable feedstocks for the production of plastics and other chemicals.
Applications of Saturated Hydrocarbons: A Wide Range of Uses
The unique properties of saturated hydrocarbons, stemming from their carbon atom arrangement and bonding, make them indispensable in numerous applications:
-
Fuels: Alkanes are the primary components of natural gas (methane, ethane) and petroleum (a mixture of alkanes, cycloalkanes, and other hydrocarbons). They are widely used as fuels for transportation, heating, and electricity generation.
-
Solvents: Certain alkanes, such as hexane and heptane, are used as solvents in various industrial processes. Their non-polar nature makes them effective solvents for non-polar substances.
-
Plastics: The cracking of alkanes yields alkenes, which are the building blocks for the production of various plastics like polyethylene and polypropylene.
-
Lubricants: Higher molecular weight alkanes are used as lubricants in engines and machinery due to their viscosity and ability to reduce friction.
-
Waxes: Long-chain alkanes are components of paraffin wax, used in candles, coatings, and other applications.
Conclusion: The Significance of Carbon Atoms in Saturated Hydrocarbon Chemistry
The properties and applications of saturated hydrocarbons are profoundly shaped by the arrangement and bonding of their carbon atoms. The tetrahedral geometry of carbon, the possibility of straight-chain and branched-chain isomers, and the impact of chain length all contribute to the diverse nature of these compounds. Understanding these fundamental aspects of saturated hydrocarbon chemistry is crucial for various scientific and industrial endeavors, from fuel production and refining to polymer synthesis and materials science. The seemingly simple structure of saturated hydrocarbons conceals a rich and complex chemistry that continues to be an area of active research and innovation. Further exploration into the intricacies of carbon-carbon and carbon-hydrogen bonding within these molecules promises to yield even more exciting advancements in the future.
Latest Posts
Latest Posts
-
Does Ionization Energy Increase Across A Period
Apr 01, 2025
-
Integration By Parts How To Choose U And Dv
Apr 01, 2025
-
Protein Synthesis Takes Place In The
Apr 01, 2025
-
Microscopic Anatomy Of A Muscle Fiber
Apr 01, 2025
-
What Is The General Equation For Cellular Respiration
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Carbon Atoms In Saturated Hydrocarbons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
