Does Ionization Energy Increase Across A Period
Muz Play
Apr 01, 2025 · 6 min read
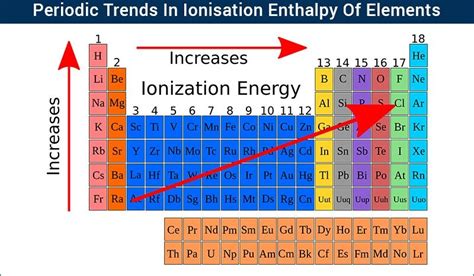
Table of Contents
Does Ionization Energy Increase Across a Period? A Comprehensive Exploration
Ionization energy, a fundamental concept in chemistry, dictates the energy required to remove an electron from a gaseous atom or ion. Understanding its trends across the periodic table is crucial for predicting chemical reactivity and behavior. This article delves deep into the question: Does ionization energy increase across a period? The answer, while seemingly simple, requires a nuanced understanding of atomic structure and the forces at play within an atom.
The Fundamental Principles: Atomic Structure and Effective Nuclear Charge
Before we explore the trend of ionization energy across a period, let's solidify our understanding of the underlying principles. An atom's structure consists of a positively charged nucleus containing protons and neutrons, surrounded by negatively charged electrons residing in various energy levels or shells. The attraction between the positively charged nucleus and the negatively charged electrons is the driving force behind the atom's stability.
The effective nuclear charge (Zeff) plays a pivotal role in determining ionization energy. Zeff represents the net positive charge experienced by an electron, considering the shielding effect of other electrons. Inner electrons shield outer electrons from the full positive charge of the nucleus. Therefore, the effective nuclear charge is always less than the actual nuclear charge (the number of protons).
As we move across a period, the number of protons in the nucleus increases, while the number of electron shells remains constant. This increase in protons leads to a stronger attraction between the nucleus and the outermost electrons. This stronger pull directly impacts the energy required to remove an electron—the ionization energy.
Ionization Energy Trend Across a Period: A Detailed Analysis
Yes, ionization energy generally increases across a period. This trend is a direct consequence of the increasing effective nuclear charge. As we move from left to right across a period, electrons are added to the same principal energy level (shell). While the shielding effect slightly increases with the addition of electrons, the increase in nuclear charge is significantly greater. This results in a higher Zeff, making it increasingly difficult to remove an electron.
Let's illustrate this with an example: Consider the elements in the second period: Lithium (Li), Beryllium (Be), Boron (B), Carbon (C), Nitrogen (N), Oxygen (O), Fluorine (F), and Neon (Ne). As we move from Li to Ne, the number of protons increases, leading to a greater effective nuclear charge. Consequently, the ionization energy increases progressively from Li to Ne.
- Lithium (Li): Possesses a relatively low ionization energy because its valence electron is shielded by the inner shell electrons.
- Neon (Ne): Exhibits the highest ionization energy in the second period because its valence electrons experience the strongest effective nuclear charge. Removing an electron from a completely filled valence shell requires significantly more energy.
Exceptions and Irregularities: A Closer Look
While the general trend of increasing ionization energy across a period is observed, certain exceptions exist. These irregularities arise due to the complexities of electron configurations and electron-electron repulsions.
One notable exception is observed between the elements Nitrogen (N) and Oxygen (O). Nitrogen has a half-filled p subshell, with three unpaired electrons. These unpaired electrons experience minimal electron-electron repulsion. However, Oxygen has one paired electron in its p subshell, leading to increased electron-electron repulsion. This repulsion slightly lowers the effective nuclear charge experienced by the outermost electron in Oxygen, leading to a slightly lower ionization energy compared to Nitrogen.
Similar irregularities can be observed in other periods, highlighting the intricate interplay between electron-electron repulsions and the effective nuclear charge.
Successive Ionization Energies: Unveiling Further Trends
The discussion so far has focused on the first ionization energy, the energy required to remove the first electron. However, it's crucial to understand successive ionization energies (the energy required to remove subsequent electrons). These energies provide further insights into atomic structure and stability.
Generally, successive ionization energies increase significantly. Removing each subsequent electron becomes progressively more difficult due to the increasing positive charge of the resulting ion and the decreasing shielding effect. The large jump in ionization energy between removing valence electrons and inner shell electrons is particularly noteworthy, indicating the stability of a completely filled valence shell.
The Significance of Ionization Energy in Chemistry
The ionization energy is a cornerstone concept in chemistry, with wide-ranging implications:
- Predicting Chemical Reactivity: Elements with low ionization energies readily lose electrons and tend to be highly reactive metals. Elements with high ionization energies tend to be unreactive nonmetals, gaining electrons rather than losing them.
- Understanding Chemical Bonding: Ionization energy helps explain the formation of ionic bonds, where electrons are transferred from one atom to another. The difference in ionization energies between the two atoms dictates the ease of electron transfer.
- Determining Oxidation States: The ionization energy plays a role in determining the various oxidation states an element can exhibit. Higher ionization energies make it less likely for an element to lose multiple electrons and exhibit higher oxidation states.
- Spectroscopic Studies: Ionization energy is directly related to the energy levels of electrons within an atom, making it an important parameter in spectroscopic studies.
Factors Influencing Ionization Energy: A Holistic Perspective
Several factors collectively influence ionization energy, creating the overall observed trends:
- Nuclear Charge: A higher nuclear charge results in a stronger attraction to electrons, increasing ionization energy.
- Atomic Radius: A larger atomic radius leads to a weaker attraction between the nucleus and outer electrons, decreasing ionization energy.
- Shielding Effect: Inner electrons shield outer electrons from the full nuclear charge, reducing the effective nuclear charge and ionization energy.
- Electron-Electron Repulsion: Repulsion between electrons in the same shell can slightly lower the effective nuclear charge and ionization energy.
- Electron Configuration: Half-filled and completely filled subshells exhibit extra stability, leading to higher ionization energies.
Conclusion: A Recap of Ionization Energy Trends Across a Period
To summarize, ionization energy generally increases across a period. This trend is primarily driven by the increasing effective nuclear charge resulting from the addition of protons without a corresponding increase in shielding electrons. However, exceptions and irregularities exist due to the complex interplay between electron-electron repulsions and electron configurations. Understanding this fundamental trend is crucial for interpreting chemical reactivity, bonding, and other related properties of elements within the periodic table. By grasping the underlying principles of atomic structure and the factors influencing ionization energy, we gain a deeper appreciation for the predictive power of the periodic table and the fundamental building blocks of matter. Further exploration into successive ionization energies unveils additional insights into atomic stability and electron configurations. The significance of ionization energy extends to various areas of chemistry, solidifying its status as a core concept in the field.
Latest Posts
Latest Posts
-
Membrane That Holds The Coils Of The Small Intestine
Apr 02, 2025
-
What Is The Conflict Of The Play
Apr 02, 2025
-
The Cell Is The Basic Unit Of
Apr 02, 2025
-
Comparison Of Somatic And Autonomic Nervous Systems Concept Map
Apr 02, 2025
-
How To Convert Molecules To Atoms
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Does Ionization Energy Increase Across A Period . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
