A Solution Is A Homogeneous Mixture
Muz Play
Mar 30, 2025 · 6 min read
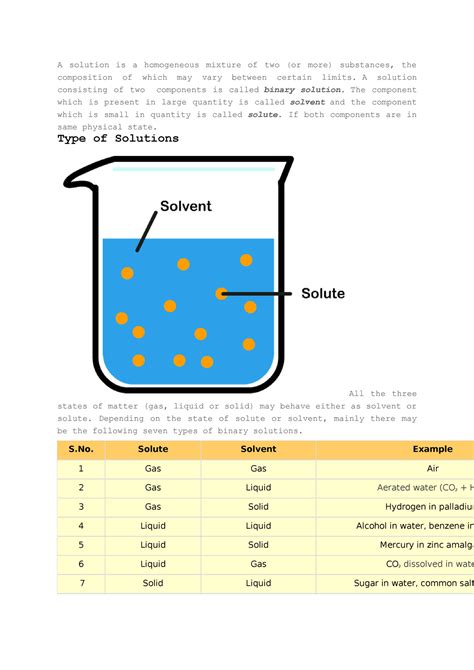
Table of Contents
A Solution is a Homogeneous Mixture: A Deep Dive into Chemistry
Understanding the fundamental concepts of chemistry is crucial for anyone pursuing scientific studies or simply seeking a deeper understanding of the world around us. One of these foundational concepts is the classification of matter, and within that classification, the concept of a solution as a homogeneous mixture holds significant importance. This article will delve deep into this topic, exploring the properties of solutions, the different types of solutions, the factors influencing solubility, and the applications of solutions in various fields.
What is a Mixture?
Before diving into solutions, let's first clarify what a mixture is. A mixture is a substance comprising two or more components that are not chemically bonded. Crucially, the components retain their individual chemical properties within the mixture. Mixtures can be easily separated into their constituent parts through physical methods, such as filtration, distillation, or evaporation. They differ significantly from compounds, where elements are chemically bonded and their properties are altered.
The Homogeneity of Solutions: A Defining Characteristic
Mixtures are categorized as either homogeneous or heterogeneous. A homogeneous mixture is one where the composition is uniform throughout. This means that the different components are evenly distributed at a microscopic level, and you wouldn't be able to visually distinguish one component from another. Solutions are quintessential examples of homogeneous mixtures.
In contrast, a heterogeneous mixture displays non-uniform composition. You can readily identify distinct regions or phases within the mixture. Think of oil and water – they form a heterogeneous mixture where the oil floats on top of the water.
Solutions: A Detailed Look
A solution is a special type of homogeneous mixture where one substance, the solute, is dissolved in another substance, the solvent. The solute is typically present in a smaller amount than the solvent. The resulting solution takes on the physical state of the solvent. For example, if you dissolve salt (solute) in water (solvent), the resulting solution is a liquid.
Key Characteristics of Solutions:
- Homogeneity: As already emphasized, solutions are completely uniform throughout.
- Transparency: True solutions are typically transparent; you can see through them.
- Particle Size: The solute particles in a solution are extremely small, typically at the atomic or molecular level. This small size is what contributes to the homogeneity and transparency of the solution.
- Stability: Solutions are generally stable, meaning the solute doesn't settle out over time. The particles remain dispersed throughout the solvent.
- Filtration: Solutions cannot be separated by simple filtration because the solute particles are too small to be trapped by filter paper.
Types of Solutions
Solutions can be categorized based on the physical states of the solute and solvent. This leads to a variety of solution types, including:
-
Solid in Liquid: This is perhaps the most common type of solution. Examples include saltwater (salt dissolved in water), sugar dissolved in water, and many metal alloys in molten form before solidification.
-
Liquid in Liquid: These solutions are formed when one liquid dissolves in another. A classic example is the miscibility of alcohol and water.
-
Gas in Liquid: Gases can also dissolve in liquids. Carbonated water is a prime example, with carbon dioxide dissolved in water under pressure.
-
Solid in Solid: Many alloys are examples of solid solutions. Steel, an alloy of iron and carbon, demonstrates this. The carbon atoms are dispersed within the iron crystal lattice.
-
Gas in Gas: Air is a classic example of a gas in gas solution. Various gases like oxygen, nitrogen, and carbon dioxide are uniformly mixed.
-
Liquid in Gas: While less common in everyday life, humidity (water vapor in air) is an example of this type of solution.
Factors Affecting Solubility
The solubility of a substance refers to its ability to dissolve in a given solvent. Several factors influence solubility:
-
Nature of Solute and Solvent: The adage "like dissolves like" is highly relevant. Polar solvents tend to dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. For example, water, a polar solvent, readily dissolves ionic compounds like salt, but it does poorly with nonpolar substances like oil.
-
Temperature: The effect of temperature on solubility varies depending on whether the dissolving process is exothermic or endothermic. For many solid solutes in liquid solvents, solubility increases with increasing temperature. However, the solubility of gases in liquids usually decreases with increasing temperature.
-
Pressure: Pressure significantly affects the solubility of gases in liquids. According to Henry's Law, the solubility of a gas is directly proportional to the partial pressure of that gas above the liquid. This is why carbonated beverages fizz when opened – the reduction in pressure allows dissolved carbon dioxide to escape.
-
Surface Area: Increasing the surface area of the solute (e.g., by using finely powdered solute) can increase the rate of dissolution. A larger surface area provides more contact points between the solute and the solvent.
Applications of Solutions
Solutions play a vital role in numerous aspects of our lives and across various scientific fields:
-
Medicine: Many drugs are administered in solution form, ensuring efficient absorption into the bloodstream. Intravenous fluids are solutions used to maintain hydration and electrolyte balance.
-
Biology: Biological systems rely heavily on solutions. Blood plasma is a solution containing various dissolved substances, including nutrients, hormones, and waste products. Cellular processes also occur within aqueous solutions.
-
Industry: Solutions are used extensively in industrial processes. Electroplating, a technique used to coat metal objects with a thin layer of another metal, involves solutions. Many chemical reactions occur in solution, facilitating the production of various chemicals and materials.
-
Agriculture: Fertilizers are often applied as solutions, allowing for better absorption by plant roots. Pesticides and herbicides are also frequently formulated as solutions for easier application.
Beyond Basic Solutions: Colloids and Suspensions
While solutions represent a homogeneous mixture, it's important to distinguish them from colloids and suspensions. These are also mixtures but differ in the size of their dispersed particles:
-
Colloids: Colloids have particles larger than those in solutions but smaller than those in suspensions. These particles don't settle out easily and scatter light, a phenomenon known as the Tyndall effect. Milk and fog are examples of colloids.
-
Suspensions: Suspensions have relatively large particles that settle out over time. They are heterogeneous mixtures, and the particles can be separated by filtration. Muddy water is a good example of a suspension.
Conclusion: The Significance of Understanding Solutions
Understanding solutions as homogeneous mixtures is a cornerstone of chemistry. The concepts explored in this article – the properties of solutions, the different types of solutions, the factors influencing solubility, and the wide range of applications – are essential for grasping the complexity and interconnectedness of the natural world. From the biological processes within our bodies to the industrial processes that shape our society, solutions play a crucial role. By gaining a deeper understanding of solutions, we unlock a better comprehension of the chemical processes that govern our world. Further exploration into advanced topics such as colligative properties, solubility product constants, and various solution equilibrium concepts will provide even richer insights into the fascinating world of homogeneous mixtures and their diverse applications.
Latest Posts
Latest Posts
-
The Atomic Radii Of The Elements In The Nitrogen Group
Apr 01, 2025
-
What Is The Purpose Of Using A Pedigree
Apr 01, 2025
-
How To Tell If A Function Is Continuous Without Graphing
Apr 01, 2025
-
How To Shift A Function To The Right
Apr 01, 2025
-
What Is The Second Step Of Photosynthesis
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about A Solution Is A Homogeneous Mixture . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
