The Atomic Radii Of The Elements In The Nitrogen Group
Muz Play
Apr 01, 2025 · 6 min read
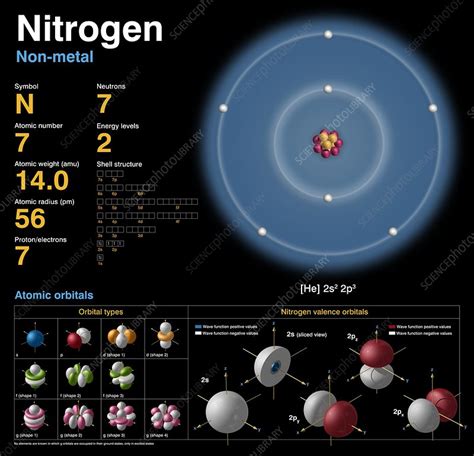
Table of Contents
The Atomic Radii of the Elements in the Nitrogen Group: A Comprehensive Exploration
The nitrogen group, also known as Group 15 or pnictogens, comprises nitrogen (N), phosphorus (P), arsenic (As), antimony (Sb), and bismuth (Bi). These elements share a common valence electron configuration of ns²np³, leading to a rich diversity in their chemical properties and, importantly, their atomic radii. Understanding the trends in atomic radii within this group is crucial for comprehending their reactivity and the properties of their compounds. This article will delve into a detailed examination of the atomic radii of these elements, exploring the factors that influence them and the consequences of these variations.
Understanding Atomic Radius
Before we embark on the specific trends in the nitrogen group, let's define what we mean by atomic radius. Atomic radius refers to the distance from the nucleus to the outermost stable electron orbital of an atom. Precisely measuring this distance is challenging, as the electron cloud doesn't have a sharply defined boundary. Consequently, different methods exist for determining atomic radius, leading to slight variations in reported values. Commonly used methods include covalent radius (half the distance between the nuclei of two identical atoms bonded covalently) and metallic radius (half the distance between adjacent nuclei in a metallic crystal lattice). We will generally discuss trends using covalent radii for consistency, acknowledging the inherent limitations of these measurements.
Trends in Atomic Radii Across the Nitrogen Group
A clear trend is observed when examining the atomic radii down the nitrogen group: atomic radius increases down the group. This increase is a consequence of several factors:
1. Increasing Principal Quantum Number (n):
As we move down the group from nitrogen to bismuth, the principal quantum number (n) of the valence electrons increases. This signifies that the valence electrons occupy progressively higher energy levels, farther from the nucleus. Consequently, the electron shells increase in size, resulting in a larger atomic radius. Nitrogen, with n=2, has a significantly smaller radius than bismuth, with n=6.
2. Shielding Effect:
The increase in the number of inner electron shells also contributes to the expansion of atomic radius. The inner electrons shield the outer valence electrons from the full positive charge of the nucleus. This shielding effect reduces the effective nuclear charge experienced by the valence electrons. As the number of inner electrons increases down the group, the shielding effect becomes more pronounced, leading to weaker attraction between the nucleus and the valence electrons, further increasing the atomic radius.
3. Electron-Electron Repulsion:
With an increasing number of electrons, the repulsion between the electrons also becomes significant. This inter-electronic repulsion counteracts the attractive force of the nucleus, thus pushing the valence electrons further away and contributing to the larger atomic radius in heavier elements.
Numerical Comparison and Data Analysis
While exact values might vary slightly depending on the measurement method, the general trend of increasing atomic radius remains consistent. A simplified representation (values are approximate and should be considered for comparative purposes only):
- Nitrogen (N): ~70 pm (picometers)
- Phosphorus (P): ~110 pm
- Arsenic (As): ~120 pm
- Antimony (Sb): ~140 pm
- Bismuth (Bi): ~150 pm
This data clearly demonstrates the substantial increase in atomic radius as we descend the nitrogen group. The difference between Nitrogen and Bismuth is striking, highlighting the significant effect of adding electron shells and increasing shielding.
Consequences of Varying Atomic Radii
The differences in atomic radii have profound implications for the chemical and physical properties of these elements:
1. Reactivity:
The larger atomic radius in heavier elements like bismuth translates to weaker attraction between the nucleus and the valence electrons. This makes it easier to remove valence electrons, leading to increased metallic character down the group. Nitrogen, with its small radius and strong hold on its valence electrons, is a non-metal, while bismuth exhibits significant metallic properties.
2. Ionization Energy:
Ionization energy, the energy required to remove an electron from an atom, is closely related to atomic radius. The ionization energy generally decreases down the group because of the increased atomic radius and reduced effective nuclear charge. This explains why bismuth is more readily ionized than nitrogen.
3. Electronegativity:
Electronegativity, the ability of an atom to attract electrons in a chemical bond, also displays a decreasing trend down the group. The larger atomic radius in heavier elements reduces their ability to attract electrons, resulting in lower electronegativity.
4. Melting and Boiling Points:
While the trend in melting and boiling points is less straightforward, the size and interatomic forces play a role. The increased metallic character down the group influences bonding types, affecting the strength of interatomic interactions. Phosphorus exhibits different allotropic forms with varying melting points, while metallic bonding in bismuth leads to relatively low melting and boiling points compared to some of its lighter counterparts.
5. Bonding Preferences:
The changes in atomic size influence the preferred bonding modes of the pnictogens. Nitrogen, being small and highly electronegative, readily forms covalent bonds, often exhibiting multiple bonds. Heavier elements, with their larger size and lower electronegativity, show a greater tendency towards metallic bonding and weaker covalent interactions.
Anomalous Behavior of Nitrogen
While the overall trend of increasing atomic radius is clear, nitrogen exhibits some anomalous behavior compared to the rest of the group. This is primarily attributed to its small atomic size and the absence of d-orbitals in its valence shell. This results in:
- Higher ionization energy and electronegativity: Nitrogen holds onto its electrons more tightly than the other elements in the group.
- Stronger tendency for multiple bonding: Nitrogen readily forms triple bonds (e.g., in N₂) due to its smaller size and high electronegativity, allowing for effective orbital overlap.
- Different bonding preferences: Nitrogen's preference for covalent bonds contrasts with the increased metallic character seen in heavier pnictogens.
- Formation of unique compounds: Nitrogen forms unique compounds like nitrous oxide (N₂O) and nitric acid (HNO₃), which are less common for the heavier pnictogens.
Conclusion
The atomic radii of the elements in the nitrogen group display a clear trend of increasing size down the group. This increase is directly linked to the addition of electron shells, increased shielding effect, and greater electron-electron repulsion. These variations in atomic radius profoundly impact the chemical and physical properties of the elements, leading to differences in reactivity, ionization energy, electronegativity, bonding preferences, and the types of compounds they form. While a consistent pattern is observed, nitrogen's unique properties, resulting from its small size and lack of readily accessible d-orbitals, create exceptions to some of the general trends. Understanding these trends and anomalies provides a crucial foundation for comprehending the diverse chemistry of the nitrogen group elements and their role in various scientific disciplines. Further research involving advanced spectroscopic techniques continues to refine our understanding of atomic dimensions and their impact on chemical behavior. The continuing exploration of the pnictogens promises exciting discoveries in materials science, catalysis, and other fields.
Latest Posts
Latest Posts
-
What Would The Potential Of A Standard Hydrogen Electrode
Apr 02, 2025
-
Draw The Tautomer Of This Aldehyde
Apr 02, 2025
-
What Is A Non Rigid Transformation
Apr 02, 2025
-
Write The Equation In Spherical Coordinates
Apr 02, 2025
-
In What Form Do Fats First Enter The Bloodstream
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about The Atomic Radii Of The Elements In The Nitrogen Group . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
