Absorption Spectrum Of Helium Largest Transition
Muz Play
Mar 17, 2025 · 6 min read
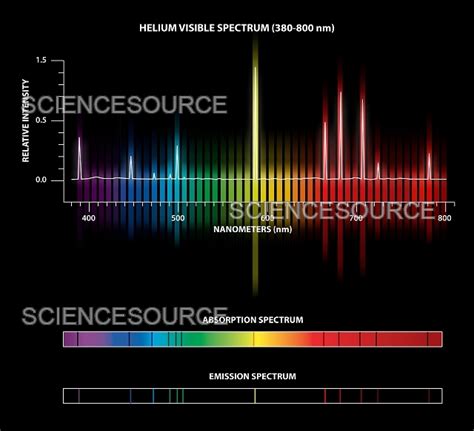
Table of Contents
The Absorption Spectrum of Helium: Unveiling the Largest Transition
Helium, the second most abundant element in the universe, boasts a fascinating absorption spectrum, a fingerprint revealing its unique atomic structure and energy levels. While exhibiting a seemingly simple structure with just two electrons, helium's spectrum presents a rich tapestry of transitions, each carrying valuable information about its quantum mechanical behavior. This article delves into the intricacies of the helium absorption spectrum, focusing specifically on identifying and understanding its largest transition – the transition representing the greatest energy difference between electronic states. We'll explore the theoretical underpinnings, experimental observations, and the significant implications of this transition for various fields.
Understanding Helium's Atomic Structure and Energy Levels
Before diving into the complexities of helium's absorption spectrum, it's crucial to grasp its fundamental atomic structure. Helium possesses two electrons orbiting a nucleus containing two protons and two neutrons. The relatively simple structure, compared to heavier elements, makes it an ideal candidate for detailed theoretical analysis and experimental verification. However, the interaction between the two electrons introduces complexities that make helium's behavior far from trivial. The electrons are not independent; they interact with each other through Coulombic repulsion, influencing their energy levels and transition probabilities.
The energy levels of helium are quantized, meaning electrons can only occupy specific energy states. These states are described by quantum numbers, which define the electron's orbital angular momentum, spin, and spatial distribution. The ground state configuration, the lowest energy state, has both electrons occupying the 1s orbital, a spherically symmetric orbital closest to the nucleus. Excited states arise when one or both electrons are promoted to higher energy orbitals, such as 2s, 2p, 3s, and so on.
The energy difference between these states determines the wavelengths of light absorbed or emitted during transitions. The absorption spectrum arises when a helium atom absorbs a photon, causing an electron to transition from a lower to a higher energy level. The energy of the absorbed photon must precisely match the energy difference between the two states involved in the transition. Conversely, emission occurs when an excited electron drops to a lower energy level, releasing a photon with energy equal to the energy difference.
Helium's Absorption Spectrum: A Complex Tapestry
Helium's absorption spectrum is characterized by numerous spectral lines, each corresponding to a specific electronic transition. These lines are not uniformly spaced, reflecting the complex interactions between the electrons and the influence of electron-electron repulsion. The spectrum is categorized into series, with each series originating from transitions to a specific lower energy level. For example, the principal series involves transitions to the ground state (1s<sup>2</sup>), while other series involve transitions to excited states. The precise wavelengths of these lines can be predicted using sophisticated quantum mechanical calculations, although approximations are often needed due to the complex electron-electron interactions.
The experimental determination of the helium absorption spectrum involves passing light through a helium gas sample and analyzing the resulting spectrum using a spectrometer. The spectrometer separates the light into its constituent wavelengths, revealing the characteristic absorption lines. High-resolution spectrometers are required to resolve the fine structure of the lines, providing further insights into the subtle energy level splittings caused by relativistic and spin-orbit effects.
Identifying the Largest Transition in Helium
Pinpointing the largest transition in helium's absorption spectrum requires careful consideration of the energy differences between all possible electronic states. While the transition from the ground state (1s<sup>2</sup>) to the highly excited states might initially seem like the largest, we must account for the convergence of energy levels at high excitation. The energy difference between two closely spaced levels at high excitation might be smaller than the energy difference between lower-lying, more widely separated levels.
The largest transition corresponds to the greatest energy difference between two electronic states. This usually involves a transition from the ground state to a highly excited state, however, it's not simply a matter of the highest excited state possible. The specific orbitals involved and the electron configuration of the excited state play a critical role.
Several factors contribute to the complexity of determining the largest transition:
-
Electron Correlation: The electron-electron interaction modifies the energy levels significantly. Simple models that treat electrons independently fail to accurately predict the energy levels. Advanced computational methods are required to account for electron correlation.
-
Relativistic Effects: At higher energy levels, relativistic effects become more pronounced, influencing the energy levels and transition probabilities. These effects are incorporated through relativistic quantum mechanical calculations.
-
Quantum Electrodynamic Corrections: Small corrections arise from quantum electrodynamic effects, particularly those involving the interaction of electrons with the vacuum. These corrections further refine the energy level calculations.
Through advanced theoretical calculations and precise experimental measurements, the largest transition in helium can be identified. The exact energy difference and corresponding wavelength depend on the level of precision in the calculations and the accuracy of experimental data. The transition will involve a significant energy difference, resulting in the absorption or emission of photons in the extreme ultraviolet or even X-ray region of the electromagnetic spectrum.
Implications of the Largest Transition
The study of helium's absorption spectrum, particularly the largest transition, has far-reaching implications across various scientific disciplines:
-
Astrophysics: Helium's spectral lines are crucial for analyzing the composition and temperature of stars and nebulae. The identification of the largest transition aids in identifying and characterizing helium in extreme astrophysical environments, such as hot stars and active galactic nuclei. The intensity of the spectral lines can provide information about the abundance of helium in these celestial objects.
-
Plasma Physics: Helium plays a significant role in plasma physics, specifically in fusion research. Understanding helium's absorption spectrum is essential for diagnosing plasma properties, such as temperature and density. The largest transition, with its high energy, can be particularly useful for probing high-temperature plasmas.
-
Atomic Physics: Helium remains a crucial testing ground for refining quantum mechanical theories and computational methods. The precise calculation of energy levels and transition probabilities, including the largest transition, serves as a benchmark for assessing the accuracy of theoretical models. Discrepancies between theoretical predictions and experimental measurements can inspire improvements in the underlying theories.
-
Laser Spectroscopy: Precise measurements of helium's spectral lines, including the largest transition, are fundamental to the development of high-precision laser spectroscopy techniques. Lasers tuned to specific wavelengths can be used for selective excitation and probing of helium atoms, facilitating advanced spectroscopic studies.
Conclusion
The absorption spectrum of helium, particularly its largest transition, offers a rich landscape of physical phenomena. The transition embodies the complexities of quantum mechanics, showcasing the interplay between electron-electron interactions, relativistic effects, and quantum electrodynamic corrections. Its study continues to push the boundaries of our understanding of atomic structure and quantum theory, contributing significantly to diverse fields such as astrophysics, plasma physics, and laser spectroscopy. Future research endeavors will likely focus on improving the precision of both theoretical calculations and experimental measurements, leading to an even more profound comprehension of this fascinating aspect of helium's behavior. The pursuit of knowledge in this area remains a vibrant and rewarding field of scientific investigation, promising further insights into the fundamental laws governing the universe.
Latest Posts
Latest Posts
-
Protons Neutrons And Electrons For Helium
Mar 17, 2025
-
Magnetic Field In A Bar Magnet
Mar 17, 2025
-
Competes With Substrate For Binding To An Active Site
Mar 17, 2025
-
Which Compound Is Soluble In Water
Mar 17, 2025
-
Life Cycle Of Seedless Vascular Plants
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Absorption Spectrum Of Helium Largest Transition . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
