Protons Neutrons And Electrons For Helium
Muz Play
Mar 17, 2025 · 6 min read
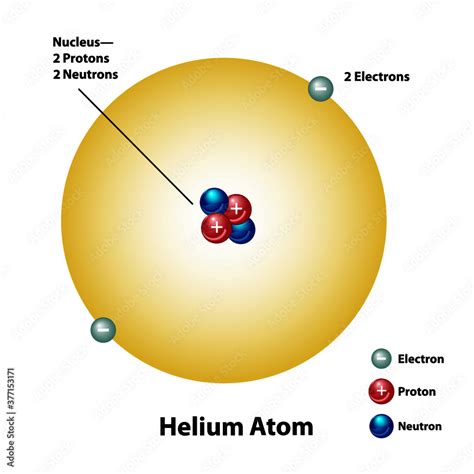
Table of Contents
Protons, Neutrons, and Electrons: Delving into the Heart of Helium
Helium, the second element on the periodic table, is a fascinating subject for exploring the fundamental building blocks of matter: protons, neutrons, and electrons. Its unique properties, stemming directly from its atomic structure, make it crucial in various applications, from cryogenics to medical imaging. This comprehensive article will dive deep into the specifics of helium's protons, neutrons, and electrons, explaining their roles, interactions, and the resulting characteristics of this remarkable element.
Understanding the Atomic Structure of Helium
Before we delve into the specifics of protons, neutrons, and electrons in helium, let's establish a foundational understanding of atomic structure. Atoms are the fundamental units of matter, composed of a central nucleus containing protons and neutrons, orbited by electrons.
- Protons: Positively charged subatomic particles found within the nucleus. The number of protons determines the element's atomic number and its identity.
- Neutrons: Neutrally charged subatomic particles residing in the nucleus alongside protons. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged subatomic particles orbiting the nucleus in specific energy levels or shells. They are significantly smaller than protons and neutrons and determine the atom's chemical properties and reactivity.
The arrangement of these subatomic particles dictates an atom's behavior and interactions with other atoms. Understanding this arrangement is crucial to understanding the properties and applications of any element, including helium.
Helium's Unique Atomic Structure: A Closer Look
Helium (He), with its atomic number of 2, possesses a remarkably simple atomic structure. This simplicity is a key factor in its unique properties.
The Nucleus: A Duo of Protons and Neutrons
The helium nucleus is composed of two protons and typically two neutrons. This combination gives helium an atomic mass number of 4 (2 protons + 2 neutrons). It's important to note that while the most common isotope of helium has two neutrons, other isotopes exist with varying numbers of neutrons. However, the two-proton configuration is constant for all helium isotopes, defining its elemental identity.
The strong nuclear force binds the protons and neutrons tightly together within the nucleus, overcoming the electrostatic repulsion between the positively charged protons. The stability of this nucleus is a significant reason why helium is an inert gas – it doesn't readily react with other elements.
The Electron Cloud: A Stable Duet
Helium's nucleus is orbited by two electrons. These electrons occupy the lowest energy level, the first electron shell, also known as the K-shell. This shell can hold a maximum of two electrons, and in helium's case, it's completely filled. This full electron shell is the primary reason for helium's inertness and chemical stability. Helium doesn't readily share, gain, or lose electrons because its outermost shell is already complete, making it extremely unreactive.
Isotopes of Helium: Variations in Neutron Count
While the number of protons remains constant at two, the number of neutrons can vary, leading to different isotopes of helium. The most common isotope, Helium-4 (⁴He), consists of two protons and two neutrons. This isotope accounts for the vast majority of helium found in nature.
Another significant isotope is Helium-3 (³He), containing two protons and only one neutron. Helium-3 is much rarer than Helium-4, but it has significant applications in scientific research, particularly in cryogenics and medical imaging. Other isotopes of helium exist, but they are highly unstable and radioactive, decaying rapidly into other elements.
The Properties of Helium: A Consequence of its Atomic Structure
Helium's unique properties are a direct consequence of its atomic structure, particularly its filled electron shell and relatively light nucleus.
Inertness and Chemical Stability
The complete outermost electron shell makes helium exceptionally inert. It rarely participates in chemical reactions because it doesn't need to gain, lose, or share electrons to achieve stability. This inertness is crucial in various applications where preventing chemical reactions is vital.
Low Density and Low Boiling Point
Helium's low atomic mass results in a very low density, making it lighter than air. This low density also contributes to its low boiling point (-268.93 °C), the lowest of all elements except for hydrogen. This extremely low boiling point makes helium an essential coolant in applications requiring extremely low temperatures, such as superconducting magnets in MRI machines.
Diffusivity and Permeability
Helium's small atomic size allows it to diffuse easily through many materials, making it useful in leak detection. Its high permeability is also exploited in applications involving gas separation and purification.
Thermal Conductivity
Helium has a relatively high thermal conductivity, meaning it efficiently transfers heat. This property makes it useful in applications requiring efficient heat dissipation, such as cooling electronic components.
Applications of Helium: Leveraging its Unique Properties
The unique properties of helium, stemming from its atomic structure, have led to its wide-ranging applications across various fields.
Cryogenics
Helium's incredibly low boiling point makes it the ideal coolant for achieving extremely low temperatures. It's used extensively in cryogenics, the science of producing and maintaining very low temperatures, which is crucial in technologies like MRI machines, superconducting magnets, and research involving superconductivity.
Scientific Research
Helium's inertness and unique properties make it an essential tool in various scientific experiments. It's used as a carrier gas in chromatography, a technique used to separate and analyze different components in a mixture. Helium-3, in particular, finds use in nuclear magnetic resonance (NMR) spectroscopy and neutron scattering experiments.
Medical Applications
Helium's low density and inertness make it suitable for use in medical imaging. It's also used as a diluent in breathing mixtures for deep-sea diving, preventing decompression sickness.
Industrial Applications
Helium's inertness, low density, and high diffusivity find applications in various industrial processes. It's used as a protective atmosphere in welding, to prevent oxidation, and in leak detection to identify microscopic holes in containers and systems.
Other Applications
Helium's applications extend to various other sectors, including aerospace (as a lifting gas in lighter-than-air craft), and as a pressurizing gas in fuel tanks and other industrial systems.
Conclusion: The Significance of Understanding Helium's Atomic Structure
Helium's simple yet unique atomic structure, characterized by its two protons, two (typically) neutrons, and two electrons, underpins its remarkable properties and widespread applications. Understanding the interplay between these subatomic particles is crucial to appreciating its inertness, low density, low boiling point, and other key characteristics that make it indispensable in diverse fields. From cryogenics and medical imaging to scientific research and industrial processes, helium's contribution is significant and its importance continues to grow as new applications are discovered. Further research into its properties and behaviors at extreme conditions will undoubtedly continue to reveal its potential for even more groundbreaking uses.
Latest Posts
Latest Posts
-
Find The Arclength Of The Curve
Mar 18, 2025
-
Which One Leaves The Solution Untouched
Mar 18, 2025
-
Which Statement Summarizes The Law Of Segregation
Mar 18, 2025
-
How Many Valence Electrons Does O3 Have
Mar 18, 2025
-
Why Do Solids Have A Definite Shape And Volume
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Protons Neutrons And Electrons For Helium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
