How Many Valence Electrons Does O3 Have
Muz Play
Mar 18, 2025 · 6 min read
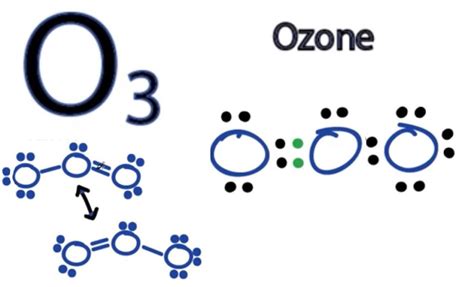
Table of Contents
How Many Valence Electrons Does O3 Have? Understanding Ozone's Bonding
Ozone (O₃), a crucial component of the Earth's atmosphere, possesses a fascinating molecular structure and electronic configuration. Understanding its valence electrons is key to grasping its chemical behavior and reactivity. This article delves deep into the question: how many valence electrons does O3 have? We'll explore the concept of valence electrons, the Lewis structure of ozone, resonance structures, formal charges, and the implications of ozone's electronic configuration for its properties.
Understanding Valence Electrons
Before tackling ozone specifically, let's establish a firm understanding of valence electrons. Valence electrons are the electrons located in the outermost shell (or energy level) of an atom. These electrons are the primary participants in chemical bonding, determining an element's reactivity and the types of bonds it can form. They dictate the atom's ability to gain, lose, or share electrons to achieve a stable electron configuration, typically resembling a noble gas (a full outermost shell).
Oxygen (O), located in Group 16 (or VIA) of the periodic table, has an atomic number of 8. This means it has 8 protons and 8 electrons. Its electron configuration is 1s²2s²2p⁴. Crucially, the outermost shell (the second shell) contains 6 electrons (2 in the 2s subshell and 4 in the 2p subshell). Therefore, oxygen has 6 valence electrons.
Determining the Total Valence Electrons in O3
Ozone (O₃) is a molecule composed of three oxygen atoms. To find the total number of valence electrons in O₃, we simply add up the valence electrons from each oxygen atom. Since each oxygen atom contributes 6 valence electrons, the total number of valence electrons in ozone is:
3 oxygen atoms × 6 valence electrons/oxygen atom = 18 valence electrons
Therefore, O₃ has a total of 18 valence electrons.
Drawing the Lewis Structure of Ozone
The Lewis structure, a visual representation of the molecule's bonding and lone pairs of electrons, is crucial for understanding ozone's electronic arrangement. Constructing the Lewis structure involves several steps:
-
Central Atom: One oxygen atom is typically placed in the center, with the other two oxygen atoms bonded to it.
-
Connecting Bonds: Single bonds are initially drawn between the central oxygen atom and the other two oxygen atoms. This uses 4 electrons (2 electrons per bond).
-
Octet Rule: We aim to satisfy the octet rule, where each atom (except hydrogen) has 8 electrons in its valence shell. After forming the initial bonds, we distribute the remaining 14 electrons (18 total - 4 used in bonds) as lone pairs on the oxygen atoms.
-
Formal Charges: Assigning formal charges helps determine the most stable Lewis structure. The formal charge is calculated as: (Valence electrons) - (Non-bonding electrons) - (1/2 Bonding electrons).
Here's where things get interesting with ozone. A single Lewis structure cannot fully represent the molecule's electronic distribution. We must consider resonance structures.
Resonance Structures in Ozone
A single Lewis structure with only single bonds and lone pairs cannot adequately depict ozone's actual bonding. Ozone exhibits resonance, meaning that the electrons are delocalized, spread across multiple bonds, rather than localized in specific positions. This is represented by drawing multiple Lewis structures, called resonance structures, that differ only in the arrangement of electrons.
We can draw two major resonance structures for ozone:
Structure 1: O=O-O (A double bond between one oxygen atom and the central oxygen atom, and a single bond between the other oxygen atom and the central oxygen atom)
Structure 2: O-O=O (A double bond between the other oxygen atom and the central oxygen atom, and a single bond between the remaining oxygen atom and the central oxygen atom)
These two structures are equivalent; the actual structure of ozone is a hybrid of these two resonance structures, with the bond order between each oxygen atom being 1.5 (average of a single and double bond).
This delocalization of electrons contributes to ozone's stability and reactivity.
Formal Charges and Resonance Stabilization
Calculating formal charges for each resonance structure helps in assessing their relative stability. The structure with the lowest formal charges on atoms is generally the most stable.
In both resonance structures of ozone, we can see that assigning formal charges minimizes the overall charge separation, increasing the stability of the molecule. The delocalization of electrons through resonance significantly lowers the energy of the molecule, contributing to its stability.
Implications of Ozone's Electronic Configuration
Ozone's 18 valence electrons, distributed across three oxygen atoms and encompassing resonance, have significant implications for its properties and reactivity:
-
Reactivity: The presence of the partially double bonds (1.5 bond order) makes ozone a strong oxidizing agent. The molecule readily accepts electrons, leading to its involvement in various chemical reactions, including the degradation of pollutants in the stratosphere.
-
Polarity: The unequal distribution of electron density due to resonance and the bent molecular geometry renders ozone a polar molecule. This polarity affects its interactions with other molecules and its solubility in polar solvents.
-
Absorption of UV Radiation: The unique electronic structure of ozone allows it to absorb high-energy ultraviolet (UV) radiation from the sun. This absorption is crucial in protecting life on Earth from the harmful effects of UV radiation. The process involves exciting electrons to higher energy levels upon UV absorption.
Ozone's Role in the Atmosphere and Environmental Impact
Ozone's role in the Earth's atmosphere is multifaceted and crucial for the planet's ecosystem:
-
Stratospheric Ozone: The ozone layer in the stratosphere absorbs most of the harmful UV-B radiation from the sun. The depletion of stratospheric ozone, primarily caused by human-made chemicals, leads to increased UV-B reaching the Earth's surface, resulting in various health and environmental problems.
-
Tropospheric Ozone: Ozone at ground level (tropospheric ozone) is a significant air pollutant, contributing to respiratory problems and harming plant life. It's a secondary pollutant, forming through chemical reactions involving nitrogen oxides and volatile organic compounds in the presence of sunlight.
Conclusion: The Significance of Ozone's 18 Valence Electrons
Ozone's 18 valence electrons are pivotal in defining its properties, structure, and role in the environment. Understanding its Lewis structure, resonance structures, and formal charges provides a comprehensive picture of this essential molecule. The delocalization of electrons through resonance contributes to its stability, while the presence of partially double bonds renders it a powerful oxidizing agent. Its role in absorbing UV radiation and its existence as both a vital atmospheric component and a harmful pollutant highlight its significance in both natural and human-influenced processes. The exploration of ozone's electronic configuration thus unveils its complex chemical behavior and profound environmental impact. The question, "How many valence electrons does O3 have?" leads us to a fascinating journey into the heart of molecular bonding and atmospheric chemistry.
Latest Posts
Latest Posts
-
Plant Cell And Animal Cell Similarities
Mar 18, 2025
-
What Is The Total Magnification Of 4x
Mar 18, 2025
-
The Cutaneous Membrane Is Blank To The Muscles
Mar 18, 2025
-
If A Hybrid Offspring Does Not Survive
Mar 18, 2025
-
5 Postulates Of Daltons Atomic Theory
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Valence Electrons Does O3 Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
