According To The Bronsted-lowry Definition A Base Is
Muz Play
Mar 16, 2025 · 6 min read
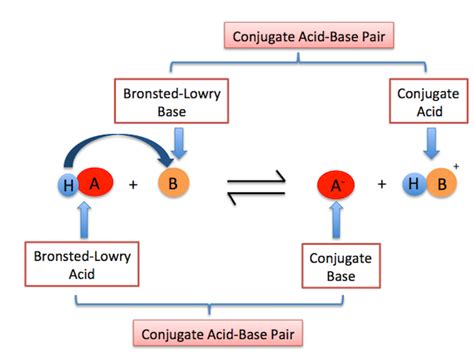
Table of Contents
According to the Brønsted-Lowry Definition, a Base Is...
The Brønsted-Lowry definition of acids and bases offers a broader and more comprehensive understanding than the earlier Arrhenius definition. While Arrhenius limited bases to hydroxide ion (OH⁻) donors, Brønsted and Lowry expanded the concept significantly, enriching our understanding of acid-base reactions across a wider range of chemical systems. This article delves deep into the Brønsted-Lowry definition of a base, exploring its nuances, applications, and implications in various chemical contexts.
Understanding the Brønsted-Lowry Definition
The cornerstone of the Brønsted-Lowry theory lies in the proton transfer. Unlike Arrhenius theory which focuses solely on the production of H⁺ and OH⁻ ions in aqueous solutions, Brønsted-Lowry theory defines acids and bases based on their ability to donate or accept protons (H⁺ ions). Specifically:
- Brønsted-Lowry Acid: A substance that donates a proton (H⁺ ion).
- Brønsted-Lowry Base: A substance that accepts a proton (H⁺ ion).
This definition significantly broadens the scope of bases. It's no longer limited to just hydroxide ions. Any species capable of accepting a proton qualifies as a Brønsted-Lowry base. This includes a vast array of molecules and ions, leading to a richer understanding of acid-base chemistry.
Key Differences from Arrhenius Definition
The Brønsted-Lowry definition transcends the limitations of the Arrhenius definition in several crucial ways:
-
Solvent Independence: The Arrhenius definition is restricted to aqueous solutions. Brønsted-Lowry, however, applies to reactions in various solvents or even without a solvent. This makes it far more versatile and applicable to a wider range of chemical scenarios.
-
Expanded Scope: The Arrhenius definition limits bases to hydroxide ion producers. The Brønsted-Lowry definition encompasses a much broader class of compounds, including many molecules and ions that don't contain hydroxide ions but can still accept protons.
-
Conjugate Acid-Base Pairs: A critical concept introduced by the Brønsted-Lowry theory is the idea of conjugate acid-base pairs. When an acid donates a proton, it forms its conjugate base. Conversely, when a base accepts a proton, it forms its conjugate acid. This relationship highlights the dynamic equilibrium inherent in many acid-base reactions.
Examples of Brønsted-Lowry Bases
The versatility of the Brønsted-Lowry definition is best illustrated through examples:
1. Hydroxide Ions (OH⁻):
The quintessential example, hydroxide ions readily accept a proton, forming water:
OH⁻(aq) + H⁺(aq) ⇌ H₂O(l)
This classic reaction falls under both Arrhenius and Brønsted-Lowry definitions.
2. Ammonia (NH₃):
Ammonia acts as a Brønsted-Lowry base by accepting a proton from water, forming ammonium ion and hydroxide ion:
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
This reaction demonstrates the base's ability to accept a proton, even without directly containing hydroxide.
3. Carbonate Ion (CO₃²⁻):
Carbonate ions are strong Brønsted-Lowry bases. They readily accept protons in reactions like:
CO₃²⁻(aq) + H⁺(aq) ⇌ HCO₃⁻(aq)
This shows the ability of polyatomic ions to function as bases by proton acceptance.
4. Water (H₂O):
Water is amphoteric, meaning it can act as both an acid and a base. It can accept a proton to form the hydronium ion (H₃O⁺):
H₂O(l) + H⁺(aq) ⇌ H₃O⁺(aq)
Or it can donate a proton, acting as an acid:
H₂O(l) ⇌ H⁺(aq) + OH⁻(aq)
This amphoteric nature highlights the versatility of the Brønsted-Lowry concept.
5. Amines (R-NH₂):
Amines, organic compounds containing a nitrogen atom with a lone pair of electrons, are common Brønsted-Lowry bases. The lone pair on nitrogen readily accepts a proton. For example, methylamine (CH₃NH₂) reacts with water as follows:
CH₃NH₂(aq) + H₂O(l) ⇌ CH₃NH₃⁺(aq) + OH⁻(aq)
The nitrogen atom’s lone pair attracts and bonds with the proton, making methylamine a Brønsted-Lowry base.
Conjugate Acid-Base Pairs: A Deeper Dive
The concept of conjugate acid-base pairs is central to the Brønsted-Lowry theory. When an acid donates a proton, the remaining species is its conjugate base. Similarly, when a base accepts a proton, the resulting species is its conjugate acid. These pairs are always linked.
Let's consider the reaction between ammonia (NH₃) and water (H₂O):
NH₃(aq) + H₂O(l) ⇌ NH₄⁺(aq) + OH⁻(aq)
In this reaction:
- NH₃ is the base, accepting a proton from water.
- NH₄⁺ is the conjugate acid of NH₃.
- H₂O is the acid, donating a proton to ammonia.
- OH⁻ is the conjugate base of H₂O.
Notice that the conjugate acid always has one more proton than its conjugate base. This relationship is fundamental in understanding acid-base equilibrium and the strength of acids and bases.
Strength of Brønsted-Lowry Bases
The strength of a Brønsted-Lowry base is determined by its ability to accept a proton. Strong bases readily accept protons, while weak bases do so less readily. This can be quantitatively assessed through the base dissociation constant (Kb). A higher Kb value indicates a stronger base.
For example, hydroxide ions (OH⁻) are strong bases, meaning they readily accept protons. On the other hand, ammonia (NH₃) is a weak base, indicating a less pronounced tendency to accept protons. This difference reflects the relative equilibrium positions of their respective reactions.
Factors influencing base strength include:
- Electronegativity: More electronegative atoms generally make a base weaker.
- Size of the atom: Larger atoms usually lead to stronger bases.
- Resonance effects: Delocalization of the negative charge through resonance stabilizes the conjugate acid and strengthens the base.
- Inductive effects: Electron-withdrawing groups generally weaken bases.
Understanding these factors helps predict the relative strength of different bases and their behavior in reactions.
Applications of Brønsted-Lowry Theory
The Brønsted-Lowry theory has far-reaching applications in various fields:
-
Analytical Chemistry: Titrations, a cornerstone of quantitative analysis, rely heavily on the principles of acid-base reactions defined by the Brønsted-Lowry theory. Determining the concentration of unknown solutions is often based on neutralization reactions involving Brønsted-Lowry acids and bases.
-
Biochemistry: Many biological processes, such as enzyme catalysis and protein folding, involve proton transfer reactions, underscoring the significance of the Brønsted-Lowry theory in understanding biological mechanisms. For example, many enzymes function by accepting or donating protons to facilitate reactions.
-
Environmental Science: Acid rain, a major environmental concern, is directly linked to the release of acidic gases that react with water in the atmosphere, forming Brønsted-Lowry acids that damage ecosystems. Understanding these reactions helps mitigate the effects of acid rain.
-
Industrial Chemistry: Numerous industrial processes rely on acid-base reactions governed by the Brønsted-Lowry theory. These include the production of various chemicals, pharmaceuticals, and materials.
-
Organic Chemistry: Many organic reactions involve proton transfer steps, making Brønsted-Lowry theory crucial in understanding reaction mechanisms and synthetic strategies.
Conclusion
The Brønsted-Lowry definition of a base significantly advanced our understanding of acid-base chemistry. Its broader scope, encompassing a wider array of compounds than the Arrhenius definition, and the introduction of conjugate acid-base pairs have proven invaluable in various scientific and technological applications. The concept of proton transfer, as a central feature of this theory, remains a vital tool in analyzing and predicting the behavior of acids and bases across diverse chemical contexts. Understanding the Brønsted-Lowry definition is thus fundamental to mastering acid-base chemistry and its multifaceted applications.
Latest Posts
Latest Posts
-
What Is A Fixed Wing Aircraft
Mar 16, 2025
-
What Is Group 17 On The Periodic Table Called
Mar 16, 2025
-
Distinguish Between Species Richness And Species Evenness
Mar 16, 2025
-
Examples Of Exponential Functions Word Problems
Mar 16, 2025
-
Competition Between Two Species Occurs When
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about According To The Bronsted-lowry Definition A Base Is . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
