What Is Group 17 On The Periodic Table Called
Muz Play
Mar 16, 2025 · 6 min read
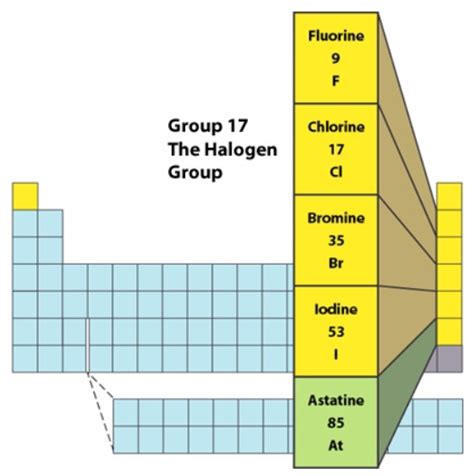
Table of Contents
What is Group 17 on the Periodic Table Called? Exploring the Halogens
Group 17 on the periodic table, also known as VIIA or 17, is famously called the halogens. The name itself, derived from Greek roots (“halos” meaning salt and “genes” meaning forming), perfectly encapsulates their defining characteristic: their tendency to react readily with metals to form salts. This article will delve deep into the fascinating world of halogens, exploring their properties, trends, uses, and the unique characteristics that set each element apart.
Understanding the Halogens: A Family Portrait
The halogens consist of five elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). Tennessine (Ts), the synthetically created element at the bottom of the group, is also technically a halogen, but its properties are less well-understood due to its short half-life and extreme rarity. All halogens share some common features, although variations exist across the group due to the periodic trends.
Key Properties of Halogens
-
Highly Reactive Nonmetals: Halogens are highly reactive nonmetals, readily accepting an electron to achieve a stable octet electron configuration. This high reactivity stems from their seven valence electrons—they are just one electron short of a full outer shell. This drives their strong tendency to form negative ions, known as halide ions (F⁻, Cl⁻, Br⁻, I⁻, At⁻).
-
Diatomic Molecules: Halogens exist as diatomic molecules in their elemental form. This means they bond to themselves to form molecules containing two atoms (e.g., F₂, Cl₂, Br₂, I₂). This is due to the strong attraction between the partially filled p-orbitals of the halogen atoms.
-
Variable Oxidation States: While predominantly forming -1 ions, halogens can exhibit various oxidation states depending on the bonding partner. This ability to exist in different oxidation states contributes to their versatility in chemical reactions. For example, chlorine can exist in oxidation states ranging from -1 to +7.
-
Trends in Properties: As we move down Group 17, distinct trends become apparent:
-
Decreasing Electronegativity: Electronegativity, the ability of an atom to attract electrons in a chemical bond, decreases down the group. Fluorine is the most electronegative element in the entire periodic table.
-
Decreasing Reactivity: Reactivity decreases down the group. While fluorine is extremely reactive, astatine's reactivity is significantly lower due to its larger atomic size and weaker attraction for electrons.
-
Increasing Atomic Radius: Atomic radius, the size of an atom, increases down the group. This is because each subsequent element adds an additional electron shell.
-
Changes in Physical State: Fluorine and chlorine are gases at room temperature, bromine is a liquid, and iodine is a solid. This reflects the increasing strength of intermolecular forces as we move down the group.
-
Individual Halogen Exploration: A Closer Look
Let's take a more in-depth look at each of the halogens, highlighting their unique properties and applications.
1. Fluorine (F): The Most Reactive Halogen
Fluorine, the lightest halogen, is a pale yellow gas that is exceptionally reactive. Its high electronegativity makes it the most reactive element on the periodic table. Fluorine's applications are diverse and significant:
-
Fluorinated Compounds: Fluorine is essential in creating numerous fluorinated compounds with various uses, including refrigerants (though many are now phased out due to environmental concerns), polymers like Teflon (polytetrafluoroethylene), and pharmaceuticals.
-
Dental Health: Fluoride, the anion of fluorine, plays a crucial role in strengthening tooth enamel and preventing tooth decay. It's commonly added to toothpaste and drinking water for dental health purposes.
-
Nuclear Energy: Fluorine compounds are used in uranium enrichment processes for nuclear power generation.
2. Chlorine (Cl): A Versatile Element
Chlorine, a greenish-yellow gas, is less reactive than fluorine but still highly reactive. Its wide range of applications underscores its importance:
-
Water Purification: Chlorine is widely used in disinfecting drinking water and swimming pools, killing harmful bacteria and viruses.
-
Industrial Chemicals: Chlorine is a crucial component in the production of various industrial chemicals, including PVC (polyvinyl chloride) plastic, solvents, and pesticides.
-
Bleaching Agent: Chlorine and its compounds are powerful bleaching agents, employed in the paper and textile industries.
3. Bromine (Br): The Liquid Halogen
Bromine is the only non-metallic element that exists as a liquid at room temperature. It's a reddish-brown liquid with a pungent odor. Its applications are somewhat less extensive than fluorine or chlorine, but still substantial:
-
Flame Retardants: Brominated flame retardants are added to plastics and textiles to improve their fire resistance. However, concerns exist regarding their environmental impact and potential health effects.
-
Agricultural Chemicals: Bromine compounds find use in agricultural chemicals such as fumigants for pest control.
-
Photography: Silver bromide is a crucial component in photographic films and papers.
4. Iodine (I): An Essential Nutrient
Iodine, a dark gray solid that sublimes readily (transitions directly from solid to gas), is an essential micronutrient for humans. Its presence is crucial for thyroid hormone production.
-
Thyroid Health: Iodine deficiency can lead to various health problems, including goiter and hypothyroidism. Iodized salt is a common method of ensuring adequate iodine intake in populations.
-
Disinfectant: Iodine and its compounds have antiseptic and disinfectant properties.
-
Organic Chemistry: Iodine and its compounds play important roles as reagents in organic chemistry reactions.
5. Astatine (At): The Radioactive Halogen
Astatine is a radioactive element, and its properties are less well-understood due to its short half-life and extreme rarity. It's found only in trace amounts, making its large-scale applications nonexistent. Its radioactive nature limits its practical use, although some research focuses on its potential applications in targeted cancer therapy.
Tennessine (Ts): A Newly Synthesized Halogen
Tennessine, the newest member of the halogen family, is a synthetically created element. Its properties are largely theoretical because of its extremely short half-life. Scientists are still actively researching this element, with only limited information about its behavior and characteristics available. Future research may unravel more about its unique attributes as a halogen.
Environmental Considerations and Safety Precautions
The reactivity of halogens makes them essential in various applications, but their handling requires careful consideration. Certain halogen compounds, particularly those containing chlorine and bromine, have raised environmental concerns:
-
Ozone Depletion: Some chlorofluorocarbons (CFCs) were notorious for depleting the ozone layer, resulting in stricter regulations and the development of ozone-friendly alternatives.
-
Water Pollution: Uncontrolled release of halogenated compounds can lead to water pollution, potentially harming aquatic life.
-
Toxicity: Certain halogen compounds can be toxic to humans and other organisms, necessitating safe handling and disposal procedures.
Conclusion: The Significance of the Halogens
Group 17, the halogens, are a fascinating group of elements with properties that have made them indispensable in many areas of modern life. From essential nutrients (iodine) to water purification (chlorine) and the creation of numerous industrial compounds (fluorine, bromine), halogens play vital roles. Understanding their properties, reactivity, and environmental impact is crucial for both maximizing their benefits and mitigating any potential risks. Continued research and development are crucial in exploring new applications and ensuring responsible use of these elements for the benefit of humanity. The study of halogens provides a microcosm of the broader wonders and complexities of the periodic table, a testament to the interconnectedness of chemistry and our world.
Latest Posts
Latest Posts
-
Fallacies Divide Into Roughly Two Kinds
Mar 17, 2025
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
-
Difference Between Chemical Reaction And Nuclear Reaction
Mar 17, 2025
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is Group 17 On The Periodic Table Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
