Difference Between Chemical Reaction And Nuclear Reaction
Muz Play
Mar 17, 2025 · 6 min read
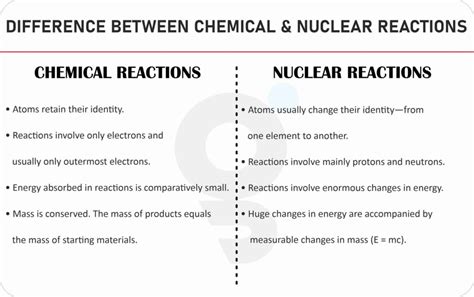
Table of Contents
Delving Deep: Chemical Reactions vs. Nuclear Reactions
Understanding the fundamental differences between chemical reactions and nuclear reactions is crucial for grasping the core principles of chemistry and physics. While both involve changes in matter, the nature of these changes, the energy involved, and the particles affected are drastically different. This comprehensive guide will illuminate these distinctions, providing a detailed exploration of each process.
What is a Chemical Reaction?
A chemical reaction involves a rearrangement of atoms and molecules to form new substances. This rearrangement occurs through the breaking and forming of chemical bonds, which are the forces that hold atoms together. Crucially, the nuclei of the atoms involved remain unchanged. Only the electrons, which occupy energy levels surrounding the nucleus, participate in the reaction.
Key Characteristics of Chemical Reactions:
- Relatively Low Energy Changes: Chemical reactions involve relatively small amounts of energy compared to nuclear reactions. The energy changes are typically measured in kilojoules (kJ) or kilocalories (kcal).
- Involvement of Electrons: Electrons are the primary players in chemical reactions. They are exchanged, shared, or rearranged to form new chemical bonds. The nucleus remains unaffected.
- Changes in Chemical Properties: Chemical reactions lead to the formation of new substances with different chemical properties. For example, the reaction between sodium (a highly reactive metal) and chlorine (a toxic gas) produces sodium chloride (table salt), a completely different substance with different properties.
- Observable Changes: Chemical reactions often produce observable changes, such as a change in color, temperature, formation of a precipitate, or the release of a gas.
- Conservation of Mass: In chemical reactions, mass is essentially conserved. The total mass of the reactants (starting materials) equals the total mass of the products (resulting substances), barring minor exceptions due to energy conversion (E=mc²).
Examples of Chemical Reactions:
- Combustion: The burning of fuels like wood or propane is a chemical reaction that releases energy in the form of heat and light.
- Rusting: The oxidation of iron in the presence of oxygen and water forms iron oxide (rust), a new substance with altered properties.
- Photosynthesis: Plants use sunlight to convert carbon dioxide and water into glucose (a sugar) and oxygen. This is a complex series of chemical reactions.
- Digestion: Our bodies utilize a series of chemical reactions to break down food into simpler molecules that can be absorbed and used for energy.
- Neutralization: The reaction between an acid and a base, such as hydrochloric acid and sodium hydroxide, produces water and a salt.
What is a Nuclear Reaction?
A nuclear reaction involves a change in the nucleus of an atom. This change can involve the emission of particles (like alpha or beta particles), the absorption of particles, or the splitting or fusing of atomic nuclei. The number of protons and neutrons in the nucleus directly influences the type of reaction and the products formed. These reactions involve significantly higher energy levels than chemical reactions.
Key Characteristics of Nuclear Reactions:
- Extremely High Energy Changes: Nuclear reactions release or absorb vast amounts of energy, typically measured in megajoules (MJ) or even gigajoules (GJ). This energy is a result of the strong nuclear force, which binds protons and neutrons together in the nucleus.
- Involvement of Protons and Neutrons: Protons and neutrons, the constituents of the atomic nucleus, are directly involved in nuclear reactions. This alters the identity of the atom itself.
- Changes in Nuclear Properties: Nuclear reactions lead to the formation of new isotopes or even entirely different elements. This is because the number of protons in the nucleus determines the element's identity.
- Radioactivity: Many nuclear reactions produce radioactive isotopes, which emit radiation as they decay into more stable forms. This radiation can be hazardous to living organisms.
- Mass-Energy Conversion: Nuclear reactions involve a significant conversion of mass into energy, as described by Einstein's famous equation, E=mc². A small amount of mass loss results in a tremendous release of energy.
Examples of Nuclear Reactions:
- Nuclear Fission: The splitting of a heavy atomic nucleus (like uranium or plutonium) into lighter nuclei, releasing a large amount of energy. This process is used in nuclear power plants and atomic bombs.
- Nuclear Fusion: The combining of light atomic nuclei (like hydrogen isotopes deuterium and tritium) into a heavier nucleus (like helium), releasing an even greater amount of energy. This process powers the sun and other stars.
- Radioactive Decay: The spontaneous transformation of an unstable atomic nucleus into a more stable one, releasing particles and/or energy in the form of radiation (alpha, beta, gamma). Examples include the decay of carbon-14 used in radiocarbon dating.
- Nuclear Transmutation: The conversion of one element into another through bombardment with particles (like neutrons or protons). This process is used to produce various radioactive isotopes for medical and industrial applications.
Comparing Chemical and Nuclear Reactions: A Detailed Table
| Feature | Chemical Reaction | Nuclear Reaction |
|---|---|---|
| Primary Change | Rearrangement of atoms and molecules | Change in atomic nuclei |
| Particles Involved | Electrons | Protons and neutrons |
| Energy Change | Relatively low (kJ or kcal) | Extremely high (MJ or GJ) |
| Mass Conservation | Approximately conserved | Not strictly conserved (mass converted to energy) |
| Element Identity | Remains unchanged | May change |
| Types of Bonds Affected | Chemical bonds | Nuclear forces |
| Observable Changes | Color change, temperature change, gas formation | Emission of radiation, nuclear transformation |
| Examples | Combustion, rusting, photosynthesis, digestion | Fission, fusion, radioactive decay, transmutation |
| Rate of Reaction | Variable, often influenced by temperature, pressure, catalysts | Often fixed, spontaneous in radioactive decay |
The Implications of these Differences
The profound differences between chemical and nuclear reactions have far-reaching implications in various fields:
- Energy Production: Nuclear reactions provide immensely more energy per unit mass than chemical reactions. This is why nuclear power plants can generate significant amounts of electricity from relatively small amounts of fuel. However, nuclear reactions also pose a greater risk of environmental contamination due to radioactive waste.
- Medicine: Radioactive isotopes produced through nuclear reactions are used in medical imaging (e.g., PET scans) and radiotherapy for cancer treatment. Chemical reactions, conversely, are fundamental to drug development, metabolism, and diagnostics.
- Industrial Processes: Both chemical and nuclear reactions are integral to various industrial processes. Chemical reactions are crucial in manufacturing plastics, fertilizers, and many other products. Nuclear reactions are used in specialized industries like nuclear medicine and some industrial sterilizations.
- Scientific Research: Understanding chemical and nuclear reactions is essential for advancing scientific knowledge in fields like chemistry, physics, materials science, and nuclear engineering. The study of these reactions aids in understanding the fundamental forces that govern the universe.
Conclusion:
Chemical and nuclear reactions are distinct processes that fundamentally differ in the nature of the changes they involve, the energy changes they produce, and the particles affected. Chemical reactions focus on electron rearrangement, leading to the formation of new substances with altered chemical properties. Nuclear reactions, on the other hand, deal with changes within the atomic nucleus, potentially resulting in the transformation of elements and the release of tremendous amounts of energy. Grasping these distinctions is essential for a comprehensive understanding of matter, energy, and their transformations in the universe. The applications of both types of reactions are ubiquitous and contribute significantly to various aspects of modern life, ranging from energy production to medicine and industrial applications. Further exploration of these fascinating fields can unlock even more profound insights into the intricate workings of the natural world.
Latest Posts
Latest Posts
-
Does Water Have High Specific Heat
Mar 17, 2025
-
What Are The Three Parameters Of Hypergeometric Pmfs
Mar 17, 2025
-
Difference Between Column And Thin Layer Chromatography
Mar 17, 2025
-
Opening A 6 Membered Ring Mechanism
Mar 17, 2025
-
What Is The Base Of A Triangle
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Difference Between Chemical Reaction And Nuclear Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
