According To The Law Of Multiple Proportions
Muz Play
Mar 15, 2025 · 6 min read
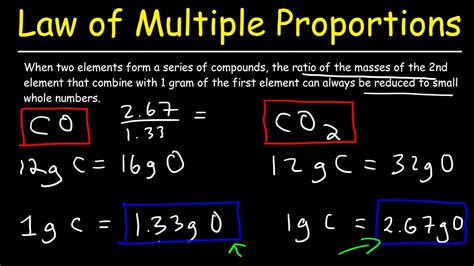
Table of Contents
According to the Law of Multiple Proportions: A Deep Dive into Chemical Composition
The Law of Multiple Proportions, a cornerstone of modern chemistry, elegantly describes the relationships between elements within different compounds. Understanding this law is crucial for grasping the fundamental principles of chemical bonding and stoichiometry. This comprehensive article will delve into the law's definition, history, examples, exceptions, and its significance in shaping our understanding of the atomic world.
Defining the Law of Multiple Proportions
The Law of Multiple Proportions states that when two elements combine to form more than one compound, the different masses of one element that combine with a fixed mass of the other element are in a simple ratio of whole numbers. In simpler terms, if two elements can form multiple compounds together, the ratios of the masses of one element that combine with a constant mass of the other element will always be simple whole number ratios. This observation directly supports the concept of atoms combining in fixed, whole-number ratios to form molecules.
This law builds upon the Law of Definite Proportions (also known as the Law of Constant Composition), which states that a given chemical compound always contains the same elements in the same proportion by mass. The Law of Multiple Proportions extends this by considering situations where the same elements can form multiple distinct compounds.
Key Aspects of the Law:
- Multiple Compounds: The law applies only when two elements form more than one compound.
- Fixed Mass: A fixed mass of one element is crucial for comparison.
- Simple Whole Number Ratio: The ratios of the masses of the other element are always simple whole numbers (e.g., 1:2, 2:3, 1:3).
- Atomic Theory Support: The law provides strong evidence for Dalton's atomic theory, which postulates that elements are composed of atoms and that atoms combine in whole number ratios.
Historical Context and John Dalton's Contribution
The Law of Multiple Proportions was proposed by John Dalton, a pioneering figure in the development of atomic theory, in 1803. His work built upon the earlier work of Joseph Proust, who formulated the Law of Definite Proportions. Dalton's meticulous experimental work, particularly his analysis of compounds containing nitrogen and oxygen, led him to observe consistent whole-number ratios in their compositions. This observation solidified his belief in the atomic nature of matter.
Dalton's insights were revolutionary, providing a quantitative framework for understanding chemical reactions and the composition of matter. Prior to Dalton's work, chemistry was largely descriptive; Dalton's law introduced a powerful predictive tool.
Illustrative Examples of the Law of Multiple Proportions
Numerous examples showcase the Law of Multiple Proportions in action. Let's examine a few key illustrations:
Example 1: Carbon Monoxide and Carbon Dioxide
Carbon and oxygen combine to form two well-known compounds: carbon monoxide (CO) and carbon dioxide (CO₂). Consider a fixed mass of carbon, say 12 grams.
- In CO, 12 grams of carbon combine with approximately 16 grams of oxygen.
- In CO₂, 12 grams of carbon combine with approximately 32 grams of oxygen.
The ratio of the masses of oxygen that combine with the fixed mass of carbon (16:32) simplifies to a simple whole-number ratio of 1:2, thus perfectly demonstrating the law.
Example 2: Nitrogen Oxides
Nitrogen and oxygen also form multiple compounds: nitrous oxide (N₂O), nitric oxide (NO), nitrogen dioxide (NO₂), and dinitrogen tetroxide (N₂O₄). Analyzing the mass ratios of oxygen that combine with a fixed mass of nitrogen in these compounds consistently reveals simple whole-number ratios, further solidifying the law's validity.
Example 3: Sulfur Oxides
Sulfur and oxygen form several oxides, including sulfur dioxide (SO₂) and sulfur trioxide (SO₃). The ratio of oxygen mass in these compounds, relative to a fixed mass of sulfur, again adheres to the simple whole-number relationship predicted by the law.
Significance and Applications of the Law
The Law of Multiple Proportions holds immense significance in chemistry for several reasons:
- Foundation of Atomic Theory: The law provided crucial evidence supporting Dalton's atomic theory, revolutionizing our understanding of matter's fundamental building blocks.
- Stoichiometry: The law forms a basis for stoichiometry, the quantitative study of chemical reactions and the relationships between reactants and products. Understanding mass ratios is fundamental to accurately predicting the amounts of substances involved in chemical processes.
- Chemical Formula Determination: The law aids in determining the empirical formulas of compounds. By analyzing the mass ratios of elements, we can deduce the simplest whole-number ratio of atoms in the compound.
- Chemical Analysis: The law plays a vital role in various chemical analysis techniques, allowing scientists to determine the composition of substances with precision.
Exceptions and Limitations
While the Law of Multiple Proportions is a powerful and widely applicable principle, it's important to acknowledge some exceptions and limitations:
- Non-Stoichiometric Compounds: Certain compounds, known as non-stoichiometric compounds or berthollides, do not adhere to the strict whole-number ratios predicted by the law. These compounds often have variable compositions due to defects in their crystal structures.
- Isotopes: The existence of isotopes, atoms of the same element with different numbers of neutrons, can slightly affect the observed mass ratios, potentially leading to deviations from simple whole numbers. However, these deviations are typically minor and do not invalidate the law's general applicability.
- Polymeric Compounds: In some polymeric compounds, the repeating units may not always adhere to simple whole-number ratios, causing deviations from the law's predictions.
Connecting to Modern Chemical Concepts
The Law of Multiple Proportions, although a product of early 19th-century chemistry, remains highly relevant in the context of modern chemical understanding. It's directly linked to:
- Mole Concept: The concept of the mole, a fundamental unit in chemistry, is inherently connected to the idea of whole-number ratios of atoms in compounds.
- Chemical Bonding: The law reinforces the concept of discrete chemical bonds formed between atoms, resulting in compounds with definite compositions.
- Spectroscopy: Modern spectroscopic techniques, such as mass spectrometry, provide precise measurements of isotopic ratios and elemental compositions, further supporting and refining our understanding of the principles embodied in the Law of Multiple Proportions.
Conclusion: A Lasting Legacy
The Law of Multiple Proportions stands as a testament to the power of observation, meticulous experimentation, and the development of insightful scientific theories. While exceptions and limitations exist, the law's fundamental principles remain a cornerstone of chemistry, providing a crucial framework for understanding the composition, reactions, and properties of matter. Its enduring legacy lies in its contribution to the development of atomic theory and its continued relevance in various branches of modern chemistry. The law serves as a powerful reminder that even seemingly simple observations can lead to revolutionary breakthroughs in our understanding of the natural world. Its simplicity belies its profound impact on the progress of science and continues to inspire future generations of chemists.
Latest Posts
Latest Posts
-
Find The Singular Values Of
May 09, 2025
-
Similarities Between The Nervous System And The Endocrine System
May 09, 2025
-
All Organic Molecules Contain The Element Carbon
May 09, 2025
-
How Doping Is Done In Semiconductor
May 09, 2025
-
Which Process Can Separate Out The Solute From The Solvent
May 09, 2025
Related Post
Thank you for visiting our website which covers about According To The Law Of Multiple Proportions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.