According To The Rules Of Osmosis A System Will
Muz Play
Mar 31, 2025 · 6 min read
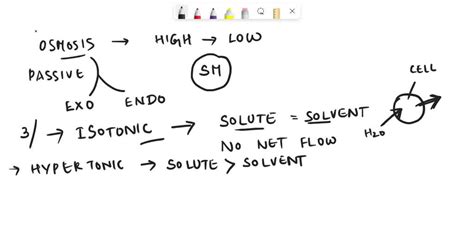
Table of Contents
According to the Rules of Osmosis, a System Will… Achieve Equilibrium
Osmosis, a fundamental process in biology and chemistry, governs the movement of water across selectively permeable membranes. Understanding its principles is crucial for comprehending various biological phenomena, from cell function to water transport in plants. This article delves deep into the rules governing osmosis, explaining how a system behaves, and exploring its implications in different contexts.
The Fundamentals of Osmosis: A Recap
Before exploring the behavior of systems under osmotic pressure, let's revisit the core principles of osmosis. Osmosis is the net movement of water molecules across a selectively permeable membrane from a region of higher water concentration to a region of lower water concentration. This movement continues until equilibrium is reached. The selectively permeable membrane allows water molecules to pass through but restricts the movement of larger solute molecules.
The driving force behind osmosis is the difference in water potential between the two regions. Water potential is a measure of the free energy of water, and water always moves from a region of higher water potential to a region of lower water potential. This movement aims to equalize the water potential across the membrane. Several factors influence water potential, most notably the concentration of solutes and the pressure applied to the system.
Key Terms to Understand:
- Selectively permeable membrane: A membrane that allows certain molecules to pass through while restricting others. Think of it as a gatekeeper, selectively allowing water but not solutes.
- Water potential: A measure of the potential energy of water. Higher water potential means water has a greater tendency to move.
- Solute: A substance dissolved in a solvent (like water) to form a solution.
- Solvent: The substance that dissolves a solute to form a solution (usually water in biological systems).
- Solution: A homogeneous mixture of a solute and a solvent.
- Osmotic pressure: The pressure required to prevent the net movement of water across a selectively permeable membrane.
Osmosis in Action: How a System Reaches Equilibrium
According to the rules of osmosis, a system will invariably strive to reach equilibrium. This equilibrium is characterized by equal water potential on both sides of the selectively permeable membrane. This doesn't necessarily mean the concentration of solutes will be equal; rather, the water potential will be equalized through the movement of water.
Let's visualize this with a simple example: Imagine two compartments separated by a selectively permeable membrane. One compartment contains pure water (high water potential), while the other contains a solution with dissolved solutes (lower water potential).
The Osmotic Process: A Step-by-Step Explanation
-
Initial State: A significant difference in water potential exists between the two compartments. The water in the pure water compartment has a higher potential to move than the water in the solute solution.
-
Water Movement: Water molecules begin to move across the membrane from the compartment with higher water potential (pure water) to the compartment with lower water potential (solute solution). This movement is driven by the tendency to equalize water potential.
-
Volume Change: As water moves into the solute solution compartment, its volume increases. The volume of the pure water compartment decreases correspondingly.
-
Pressure Build-up: The influx of water into the solute solution compartment creates pressure. This pressure is the osmotic pressure.
-
Equilibrium: The movement of water continues until the water potential becomes equal on both sides of the membrane. At this point, the net movement of water ceases, and the system has reached equilibrium. The osmotic pressure at this point is equal to the difference in water potential between the two initial compartments.
Different Osmotic Environments: Hypotonic, Isotonic, and Hypertonic Solutions
The behavior of a cell in different solutions is a classic illustration of osmotic principles. The terms hypotonic, isotonic, and hypertonic describe the relative concentrations of solute within and outside a cell.
1. Hypotonic Solution:
A hypotonic solution has a lower solute concentration compared to the inside of a cell. This means it has a higher water potential. Consequently, water moves into the cell, causing it to swell and potentially lyse (burst) if the osmotic pressure becomes too high. This is a particularly important consideration for animal cells, which lack a rigid cell wall to protect them from bursting. Plant cells, however, are generally better suited to handle hypotonic environments because their cell walls provide structural support. The cell will become turgid (firm).
2. Isotonic Solution:
An isotonic solution has the same solute concentration as the inside of the cell. In this case, the water potential is equal inside and outside the cell. Therefore, there is no net movement of water, and the cell maintains its normal shape and size. This is generally the ideal environment for animal cells.
3. Hypertonic Solution:
A hypertonic solution has a higher solute concentration compared to the inside of a cell. This means it has a lower water potential. Water moves out of the cell, causing it to shrink and plasmolyze (the cytoplasm pulls away from the cell wall in plant cells). This can be detrimental to cell function as the cell's volume decreases and its internal structures become compromised.
Practical Applications of Osmosis: Real-World Examples
Osmosis plays a vital role in numerous biological and industrial processes:
1. Water Uptake in Plants:
Plants rely heavily on osmosis to absorb water from the soil through their root hairs. The soil solution is typically hypotonic to the cells of the root hairs, leading to water uptake. This water is then transported throughout the plant via the xylem vessels, facilitating growth and maintaining turgor pressure.
2. Water Regulation in Animals:
Animals maintain their internal water balance through various mechanisms involving osmosis. The kidneys play a crucial role in regulating the concentration of solutes in the blood, ensuring that the blood remains isotonic. This prevents excessive water loss or gain.
3. Food Preservation:
Osmosis is utilized in food preservation techniques like salting and sugaring. These processes create hypertonic environments that draw water out of microorganisms, inhibiting their growth and preventing spoilage.
4. Water Purification:
Reverse osmosis, a process that uses pressure to overcome osmotic pressure, is used to purify water by removing dissolved salts and other impurities.
5. Medical Applications:
Osmosis plays a critical role in various medical applications, including intravenous fluid administration. The concentration of fluids must be carefully controlled to prevent damage to cells due to osmotic imbalances.
Beyond Basic Osmosis: Factors Influencing Osmotic Movement
While the basic principles of osmosis are relatively straightforward, several factors can influence the rate and extent of water movement:
-
Temperature: Higher temperatures generally increase the rate of osmosis. This is because increased kinetic energy increases the movement of water molecules.
-
Membrane Permeability: The permeability of the membrane to water significantly affects the rate of osmosis. More permeable membranes allow for faster water movement.
-
Surface Area: A larger surface area of the membrane increases the rate of osmosis. More membrane means more points for water molecules to cross.
-
Thickness of Membrane: A thinner membrane facilitates faster osmosis.
-
Pressure Gradient: Applying pressure to the system can influence osmosis. This is the basis for reverse osmosis.
Conclusion: The Ubiquity of Osmosis
Osmosis is a fundamental process that underpins many vital biological functions and industrial applications. Understanding its principles – the movement of water from regions of higher to lower water potential until equilibrium is reached – is essential for comprehending diverse phenomena, from cell function to water purification. The concepts of hypotonic, isotonic, and hypertonic solutions, along with an awareness of the factors affecting osmotic movement, provide a comprehensive understanding of this ubiquitous process. Further research into the nuances of osmotic pressure and its implications will continue to advance our understanding of biological systems and drive innovations in diverse fields.
Latest Posts
Latest Posts
-
What Is The Chromosomal Basis Of Inheritance
Apr 02, 2025
-
Two Different Ionic Compounds Each Contain
Apr 02, 2025
-
Part Ii Equilibria Involving Sparingly Soluble Salts
Apr 02, 2025
-
Adding Strong Acid To A Buffer
Apr 02, 2025
-
The Most Reactive Group In The Periodic Table
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about According To The Rules Of Osmosis A System Will . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
