Adding And Subtracting Significant Figures Practice
Muz Play
Mar 23, 2025 · 5 min read
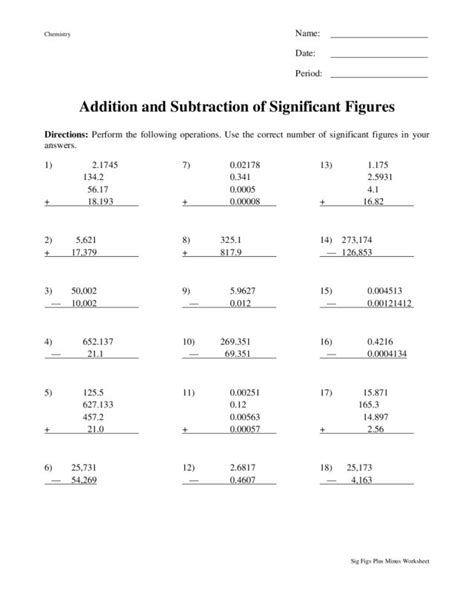
Table of Contents
- Adding And Subtracting Significant Figures Practice
- Table of Contents
- Mastering Significant Figures: A Comprehensive Guide to Addition and Subtraction
- Understanding Significant Figures: A Foundation
- Addition and Subtraction with Significant Figures: The Rules
- Advanced Scenarios and Practical Applications
- Practice Problems: Sharpening Your Skills
- Conclusion: Accuracy in the Digital Age
- Latest Posts
- Latest Posts
- Related Post
Mastering Significant Figures: A Comprehensive Guide to Addition and Subtraction
Significant figures are the backbone of accurate scientific reporting. They represent the level of precision in a measurement, indicating which digits are reliably known and which are uncertain. Understanding and correctly applying rules for significant figures is crucial for anyone working with numerical data, particularly in fields like chemistry, physics, and engineering. This comprehensive guide delves into the intricacies of adding and subtracting numbers while maintaining the integrity of significant figures. We'll move beyond simple memorization and explore the underlying principles, ensuring you develop a deep understanding of this essential skill.
Understanding Significant Figures: A Foundation
Before we tackle addition and subtraction, let's solidify our understanding of what constitutes a significant figure. Significant figures are all the digits that are known with certainty plus one uncertain digit. This uncertain digit represents the limit of the measuring instrument's precision.
Key Rules for Identifying Significant Figures:
- Non-zero digits are always significant. For example, in the number 234, all three digits are significant.
- Zeros between non-zero digits are significant. In 1005, all four digits are significant.
- Leading zeros (zeros to the left of the first non-zero digit) are not significant. 0.0025 only has two significant figures (2 and 5).
- Trailing zeros (zeros to the right of the last non-zero digit) are significant only if the number contains a decimal point. 1200 has two significant figures, while 1200.0 has five significant figures.
- Trailing zeros in a number without a decimal point are ambiguous and should be avoided. Scientific notation offers a clear way to address this ambiguity.
Scientific Notation: A Powerful Tool
Scientific notation provides a standardized way to represent numbers, especially those with many zeros. It expresses a number as a coefficient (a number between 1 and 10) multiplied by a power of 10. For example, 1200 can be expressed as 1.2 x 10³ (two significant figures) or 1.200 x 10³ (four significant figures). This clarifies the number of significant figures unambiguously.
Addition and Subtraction with Significant Figures: The Rules
The rules for adding and subtracting numbers with significant figures differ slightly from those for multiplication and division. The key concept here is to focus on the position of the last significant digit (the least precise digit).
The Rule: The result of addition or subtraction should be rounded to the same number of decimal places as the measurement with the fewest decimal places.
Let's illustrate this with examples:
Example 1:
- 12.345 g + 2.1 g = ?
In this example, 12.345 g has three decimal places, while 2.1 g has only one decimal place. The result of the addition is 14.445 g. However, we must round this to one decimal place to match the least precise measurement. Therefore, the answer is 14.4 g.
Example 2:
- 105.7 cm - 2.34 cm = ?
105.7 cm has one decimal place, and 2.34 cm also has two decimal places. The result of the subtraction is 103.36 cm. We round the answer to one decimal place, giving us 103.4 cm.
Example 3:
- 12500 kg + 320 kg = ?
Both numbers have potentially ambiguous significant figures. This is where scientific notation becomes essential for clarity: * 12500 kg = 1.25 x 10⁴ kg (three significant figures) * 320 kg = 3.2 x 10² kg (two significant figures)
Adding these in scientific notation might be cumbersome, it is better to solve the problem as it is to avoid confusion. The result is 12820 kg. Considering that 12500 is ambiguous we must round the number to two significant digits to match 320. Considering that 12500 kg is more precise than 320 kg, the uncertainty lies in the last significant figures. Therefore, the result is 1.3 x 10⁴ kg.
Example 4 (Dealing with Ambiguity):
- 100 + 0.5 =?
While the result might seem straightforward (100.5), the number 100 is ambiguous. If it represents 100.0 (three significant figures), the answer would remain 100.5. But if 100 indicates only one significant figure, the answer would need to be rounded to 100. Scientific notation could eliminate such ambiguity (1.0 x 10² + 0.5).
Advanced Scenarios and Practical Applications
Let's explore more complex scenarios to solidify your understanding:
Scenario 1: Multiple Additions/Subtractions
When performing multiple additions or subtractions, maintain the decimal place rule at each step. Don't round until the final answer. Consider this:
15.23 + 4.1 - 2.015 = ?
- Add 15.23 and 4.1: 19.33
- Subtract 2.015 from 19.33: 17.315
Since 4.1 has only one decimal place, the final answer should be rounded to one decimal place: 17.3.
Scenario 2: Dealing with Extremely Large or Small Numbers
Here, scientific notation is your best friend. It simplifies calculations and ensures you don't lose track of significant figures during calculations.
Scenario 3: Real-World Applications
Significant figures are not just an academic exercise. Their practical applications are widespread:
- Chemical Analysis: Calculations involving molarity, concentration, and stoichiometry require meticulous attention to significant figures to ensure accurate results.
- Engineering and Construction: Precise measurements and calculations are crucial for building structurally sound and functional structures.
- Medical Science: Accurate dosage calculations are vital for patient safety. Errors in significant figures can have serious consequences.
- Data Analysis and Statistics: Understanding significant figures is crucial for interpreting statistical results and drawing accurate conclusions from data.
Practice Problems: Sharpening Your Skills
Let's test your understanding with some practice problems:
- 25.67 g + 10.0 g - 3.2 g = ?
- 0.005 m + 1.2 m - 0.025 m = ?
- 125000 km + 3000 km = ? (Express your answer in scientific notation, assuming the ambiguity in 125000)
- 678.90 mL - 23.1 mL + 5.78 mL = ?
- 1.234 x 10⁻⁵ g + 2.5 x 10⁻⁶ g = ? (Express answer in scientific notation)
Solutions: (Try these on your own before checking!)
- 32.5 g
- 1.2 m
- 1.3 x 10⁵ km
- 661.6 mL
- 1.48 x 10⁻⁵ g
Conclusion: Accuracy in the Digital Age
The meticulous application of significant figures ensures that the results of calculations reflect the actual precision of the measurements involved. While seemingly simple, mastering these rules is essential for accuracy and consistency in scientific and technical work. Understanding the principles behind the rules, rather than simply memorizing them, is key to avoiding common errors and ensuring your work meets the highest standards of precision and clarity. Remember that scientific notation is a powerful tool for handling ambiguous numbers and maintaining precision, especially when dealing with extremely large or small values. By consistently applying these principles, you enhance your ability to analyze data accurately and communicate your findings effectively.
Latest Posts
Latest Posts
-
What Does Triangle Mean In Physics
Mar 26, 2025
-
Moment Of Inertia For A Uniform Rod
Mar 26, 2025
-
In Dimensional Analysis What Is A Conversion Factor
Mar 26, 2025
-
How Can You Identify Sedimentary Rocks
Mar 26, 2025
-
The Structure Of Atoms Answer Key
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Adding And Subtracting Significant Figures Practice . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
