Addition Of Water To An Alkyne
Muz Play
Mar 24, 2025 · 5 min read
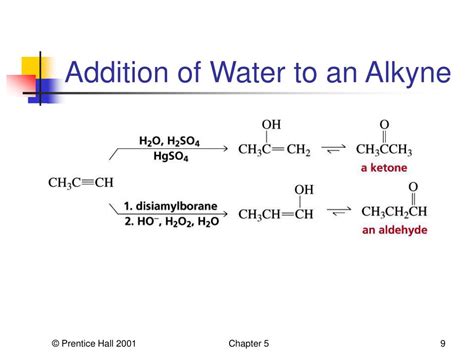
Table of Contents
The Hydration of Alkynes: A Deep Dive into Mechanisms, Regioselectivity, and Applications
The addition of water to alkynes, also known as alkyne hydration, is a fundamental organic chemistry reaction with significant implications in the synthesis of carbonyl compounds. This process transforms a relatively unreactive alkyne into a valuable ketone or aldehyde, depending on the alkyne's structure and reaction conditions. This comprehensive guide will delve into the intricacies of alkyne hydration, examining its mechanisms, regioselectivity, and diverse applications in various fields.
Understanding the Reaction: Mechanisms and Regioselectivity
Alkyne hydration doesn't proceed spontaneously under standard conditions. It requires a catalyst, typically a strong acid like sulfuric acid (H₂SO₄) or mercury(II) sulfate (HgSO₄), along with water. The reaction proceeds through a Markovnikov addition mechanism, meaning that the hydroxyl group (–OH) preferentially adds to the more substituted carbon atom of the alkyne. Let's break down the mechanism:
The Mercuric Ion-Catalyzed Hydration
This is the classic approach, utilizing mercury(II) salts as catalysts. The mechanism involves several key steps:
-
Mercuration: The alkyne initially reacts with the mercuric ion (Hg²⁺), forming a mercurinium ion intermediate. This intermediate is a three-membered ring containing mercury, and it's crucial for the regioselectivity of the reaction. The more substituted carbon atom bears a greater positive charge in the transition state, favoring the attack of the nucleophile (water) at that site.
-
Nucleophilic Attack: A water molecule acts as a nucleophile, attacking the more substituted carbon of the mercurinium ion. This step opens the ring, resulting in a neutral organomercury compound.
-
Proton Transfer: A proton transfer occurs, leading to the formation of an enol intermediate. Enols are unstable isomers of ketones and aldehydes.
-
Tautomerization: The enol tautomerizes (rearrangement) to form the more stable keto or aldehyde product. This tautomerization is usually rapid and spontaneous.
The key takeaway here is the Markovnikov regioselectivity: the hydroxyl group ends up on the more substituted carbon. For example, hydration of propyne (CH₃-C≡CH) yields acetone (CH₃-CO-CH₃), not propanal (CH₃-CH₂-CHO).
Acid-Catalyzed Hydration (without Mercury)
While mercury-catalyzed hydration is traditional, its environmental toxicity has spurred research into mercury-free alternatives. Acid-catalyzed hydration, although often less efficient, provides a greener route. The mechanism is still based on Markovnikov addition but without the mercurinium ion intermediate. Instead, a carbocation intermediate is formed, leading to the same regioselectivity outcome. The reaction generally requires higher temperatures and longer reaction times compared to the mercuric ion-catalyzed approach.
Factors Affecting Regioselectivity and Yield
Several factors can influence the outcome of alkyne hydration:
- Catalyst Choice: The choice of catalyst (HgSO₄ vs. strong acid) significantly impacts the reaction rate and yield.
- Substrate Structure: The structure of the alkyne, specifically the substitution pattern, dictates the regioselectivity and the type of carbonyl product formed. Symmetrical alkynes produce a single ketone, while unsymmetrical alkynes yield ketones according to Markovnikov's rule.
- Reaction Conditions: Temperature, concentration of reactants, and solvent choice play crucial roles in optimizing the reaction. Higher temperatures can increase the reaction rate but may also lead to side reactions.
- Steric Hindrance: Bulky substituents on the alkyne can influence the regioselectivity by hindering the approach of the nucleophile.
Applications of Alkyne Hydration
The products of alkyne hydration—ketones and aldehydes—are versatile building blocks in organic synthesis and find applications in various fields:
Pharmaceutical Industry
Ketones and aldehydes synthesized via alkyne hydration serve as vital intermediates in the production of numerous pharmaceuticals. They can be incorporated into drug molecules, enhancing their properties or facilitating their synthesis.
Polymer Chemistry
Alkyne hydration products play a critical role in the polymerization process, forming the foundation for various polymers used in materials science and engineering. These polymers find applications in diverse areas, from plastics to coatings.
Flavor and Fragrance Industry
Many naturally occurring flavor and fragrance compounds possess carbonyl functionalities. Alkyne hydration offers a valuable pathway to synthesize such compounds, contributing to the creation of artificial flavors and fragrances used in food, cosmetics, and perfumes.
Fine Chemicals Synthesis
The versatility of ketones and aldehydes obtained from alkyne hydration makes them essential intermediates in the synthesis of a wide range of fine chemicals, including dyes, pigments, and other specialty chemicals.
Beyond Simple Hydration: Variations and Extensions
The fundamental alkyne hydration reaction can be modified to achieve greater control over the reaction outcome and access a broader range of products.
Hydroboration-Oxidation
This method, employing borane (BH₃) followed by oxidation with hydrogen peroxide (H₂O₂), provides an anti-Markovnikov addition of water to alkynes. This means the hydroxyl group adds to the less substituted carbon, offering a complementary strategy to the standard Markovnikov addition.
Enantioselective Hydration
Recent advancements have focused on developing enantioselective hydration methods, which generate chiral carbonyl compounds with high enantiomeric purity. These are highly valuable in pharmaceutical and fine chemical synthesis where chirality is crucial. This often employs catalytic systems with chiral ligands.
Conclusion: A Powerful Synthetic Tool
The addition of water to alkynes is a powerful and versatile reaction in organic synthesis. Understanding the mechanisms, influencing factors, and various modifications allows chemists to selectively produce ketones and aldehydes, valuable intermediates in various industries. While the traditional mercury-catalyzed method provides a high yield and selectivity, the drive towards greener chemistry necessitates the exploration and optimization of mercury-free alternatives. Future research will likely focus on developing more efficient, environmentally friendly, and enantioselective methods for alkyne hydration, expanding the scope of this fundamental reaction even further. The continued exploration of this reaction will undoubtedly lead to new advancements in the synthesis of complex molecules and further diversify its applications in various scientific and technological fields. The ability to precisely control the regioselectivity and stereochemistry makes alkyne hydration an indispensable tool in the synthetic chemist's arsenal.
Latest Posts
Latest Posts
-
Graphs Of Sine And Cosine Functions Answer Key
Mar 25, 2025
-
Newspapers During The Revolutionary War Period Tended To
Mar 25, 2025
-
Are Ketones Or Aldehydes More Reactive
Mar 25, 2025
-
A Unicellular Protist Is Part Of Which Domain
Mar 25, 2025
-
A Species With 12 Protons And 10 Electrons Is
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Addition Of Water To An Alkyne . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
