Are Ketones Or Aldehydes More Reactive
Muz Play
Mar 25, 2025 · 6 min read
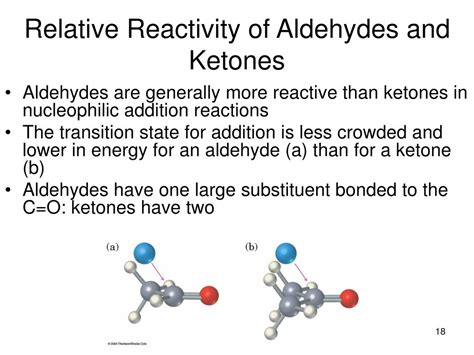
Table of Contents
Ketones vs. Aldehydes: Unveiling the Reactivity Differences
The carbonyl group (C=O), a fundamental functional group in organic chemistry, is present in both aldehydes and ketones. However, despite this shared structural feature, aldehydes and ketones exhibit significant differences in their reactivity. This article delves deep into the reasons behind these differences, exploring the factors that influence their reactivity and providing specific examples to illustrate the contrasting behavior of these two carbonyl compound classes.
Understanding the Carbonyl Group's Reactivity
The reactivity of both aldehydes and ketones stems from the polar nature of the carbonyl group. The carbon atom in the carbonyl group carries a partial positive charge (δ+), making it susceptible to nucleophilic attack. Simultaneously, the oxygen atom carries a partial negative charge (δ-), making it prone to electrophilic attack. However, the differing steric and electronic environments surrounding the carbonyl group in aldehydes and ketones lead to variations in their reactivity.
Steric Hindrance: A Key Differentiator
One major factor influencing the relative reactivity is steric hindrance. In aldehydes, the carbonyl group is bonded to one alkyl or aryl group and one hydrogen atom. This creates less steric crowding around the carbonyl carbon, making it more accessible to nucleophiles. In contrast, ketones possess two alkyl or aryl groups bonded to the carbonyl carbon. These bulky groups create greater steric hindrance, making the carbonyl carbon less accessible and thus less reactive towards nucleophilic attack. This is a significant contributor to the generally lower reactivity observed in ketones compared to aldehydes.
Electronic Effects: The Role of Inductive and Resonance Effects
Beyond steric factors, electronic effects also play a crucial role. Alkyl groups exert a positive inductive effect (+I effect), donating electron density to the carbonyl carbon. In ketones, the presence of two alkyl groups enhances this +I effect, slightly reducing the electrophilicity of the carbonyl carbon. This less electrophilic carbonyl carbon in ketones makes it a less attractive target for nucleophilic attack. Aldehydes, having only one alkyl group or a hydrogen atom, experience a weaker +I effect, resulting in a more electrophilic carbonyl carbon and increased reactivity towards nucleophiles.
Resonance effects can also subtly influence reactivity. Although less pronounced than inductive effects, resonance can slightly stabilize the carbonyl group. The extent of resonance stabilization can vary depending on the nature of the alkyl or aryl groups attached to the carbonyl carbon. This variation can contribute, albeit minimally compared to steric and inductive effects, to the reactivity differences between aldehydes and ketones.
Specific Reactions Illustrating Reactivity Differences
Let's examine some specific reactions to highlight the contrasting behavior of aldehydes and ketones:
1. Oxidation:
-
Aldehydes: Aldehydes are easily oxidized to carboxylic acids. This is a crucial difference, as ketones resist oxidation under similar conditions. Mild oxidizing agents like Tollens' reagent (ammoniacal silver nitrate) and Fehling's solution readily oxidize aldehydes, producing a silver mirror or a red precipitate of cuprous oxide, respectively. These reactions are commonly used as qualitative tests to distinguish aldehydes from ketones.
-
Ketones: Ketones are significantly more resistant to oxidation. Strong oxidizing agents and harsh conditions are required to oxidize ketones, usually resulting in the cleavage of carbon-carbon bonds, leading to a mixture of carboxylic acids.
2. Nucleophilic Addition:
-
Aldehydes and Ketones: Both aldehydes and ketones undergo nucleophilic addition reactions. However, the rate of reaction is generally faster for aldehydes due to the reasons discussed above (less steric hindrance and weaker +I effect). Examples of nucleophilic addition reactions include the reaction with Grignard reagents, organolithium reagents, and hydride reducing agents like sodium borohydride (NaBH₄) and lithium aluminum hydride (LiAlH₄). While both aldehydes and ketones react with these reagents, aldehydes react faster and generally provide higher yields.
-
Cyanohydrin Formation: This is a classic example of nucleophilic addition. Hydrogen cyanide (HCN) adds to the carbonyl group of both aldehydes and ketones to form cyanohydrins. Again, aldehydes react faster due to reduced steric hindrance.
3. Aldol Condensation:
-
Aldehydes: Aldehydes readily undergo aldol condensation, a reaction involving the addition of an enolate ion to another aldehyde molecule, followed by dehydration to form an α,β-unsaturated aldehyde. This reaction is particularly favorable for aldehydes due to their higher reactivity compared to ketones.
-
Ketones: Ketones can also undergo aldol condensation, but the reaction is slower and often requires stronger base conditions. Moreover, the self-condensation of ketones is less favored than the cross-aldol condensation (reaction between an aldehyde and a ketone).
4. Cannizzaro Reaction:
- Aldehydes: Aldehydes lacking α-hydrogens can undergo the Cannizzaro reaction, a disproportionation reaction where one molecule of the aldehyde is oxidized to a carboxylic acid while another is reduced to an alcohol. This reaction is characteristic of aldehydes and doesn't occur with ketones.
5. Wittig Reaction:
- Aldehydes and Ketones: Both aldehydes and ketones react with Wittig reagents (phosphorus ylides) to form alkenes. However, the reactivity difference remains, with aldehydes exhibiting faster reaction rates.
Summary of Reactivity Differences
| Feature | Aldehydes | Ketones |
|---|---|---|
| Steric Hindrance | Lower | Higher |
| Inductive Effect | Weaker +I effect | Stronger +I effect |
| Oxidation | Easily oxidized to carboxylic acids | Resistant to oxidation; requires harsh conditions |
| Nucleophilic Addition | Faster reaction rates | Slower reaction rates |
| Aldol Condensation | Readily undergoes self-condensation | Slower and often requires stronger base |
| Cannizzaro Reaction | Undergoes reaction (if no α-hydrogen) | Does not undergo the reaction |
Factors Affecting Reactivity Beyond the Basics
The reactivity differences discussed above are generalizations. The actual reactivity of a specific aldehyde or ketone can be further influenced by several factors:
-
Electronic effects of substituents: The nature of the alkyl or aryl groups attached to the carbonyl group can significantly alter the electron density around the carbonyl carbon, affecting its reactivity. Electron-withdrawing groups reduce reactivity, while electron-donating groups increase it.
-
Solvent effects: The solvent used for the reaction can influence the reactivity by affecting the stabilization of the reactants and transition states. Polar solvents often favor reactions with charged species.
-
Temperature and pressure: Reaction conditions such as temperature and pressure can also significantly influence the reaction rate.
Conclusion
In summary, while both aldehydes and ketones possess a carbonyl group and participate in similar reactions, aldehydes generally exhibit higher reactivity than ketones. This difference in reactivity is primarily attributed to the lower steric hindrance around the carbonyl carbon in aldehydes and the weaker +I effect exerted by the single alkyl or hydrogen substituent. Understanding these fundamental differences is crucial for predicting and controlling the outcome of reactions involving aldehydes and ketones in various synthetic organic chemistry applications. The examples provided in this article highlight the clear distinctions in their behavior and provide a strong foundation for further exploration of carbonyl chemistry. Further research into specific reactions and the influences of substituents, solvents, and reaction conditions will provide a more nuanced understanding of the fascinating world of aldehyde and ketone reactivity.
Latest Posts
Latest Posts
-
Question Lexan Draw The Monomer Used To Make This Polymer
Mar 26, 2025
-
How Do You Calculate Index Numbers
Mar 26, 2025
-
What Type Of Bonding Involves The Unequal Sharing Of Electrons
Mar 26, 2025
-
What Is A Calorie In Chemistry
Mar 26, 2025
-
Gramatica A The Verb Gustar Worksheet Answers
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Are Ketones Or Aldehydes More Reactive . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
