What Type Of Bonding Involves The Unequal Sharing Of Electrons
Muz Play
Mar 26, 2025 · 7 min read
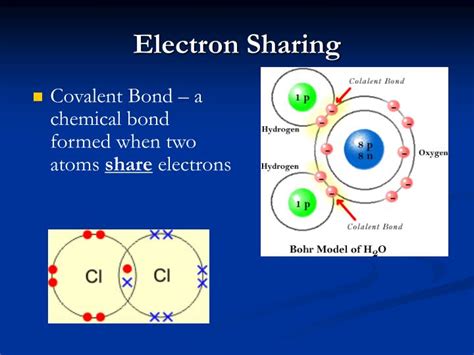
Table of Contents
What Type of Bonding Involves the Unequal Sharing of Electrons?
Polar covalent bonding is the type of bonding that involves the unequal sharing of electrons between atoms. This unequal sharing arises from a difference in electronegativity between the atoms involved. Understanding polar covalent bonds is crucial to comprehending the properties and behavior of a vast array of molecules, from simple diatomic molecules to complex biological macromolecules. This article delves into the intricacies of polar covalent bonding, exploring its underlying principles, its impact on molecular properties, and its importance in various chemical contexts.
Understanding Electronegativity: The Driving Force Behind Polarity
Before we delve into the specifics of polar covalent bonding, it's crucial to understand the concept of electronegativity. Electronegativity is a measure of an atom's ability to attract electrons towards itself within a chemical bond. Different elements possess different electronegativities, reflecting their atomic structure and electron configurations. Elements on the right side of the periodic table, particularly those in Groups 16 and 17 (the halogens and chalcogens), generally exhibit higher electronegativities than those on the left. This is because they have a greater nuclear charge relative to their number of electron shells, resulting in a stronger pull on shared electrons.
The Electronegativity Scale
The electronegativity of elements is often represented on a scale, with fluorine (F) assigned the highest value of 4.0. Other elements are then assigned values relative to fluorine. While various electronegativity scales exist (Pauling scale, Mulliken scale, Allred-Rochow scale), the Pauling scale remains the most widely used and is referenced throughout this article. Knowing the electronegativity values of atoms allows us to predict the type of bond that will form between them.
The Formation of Polar Covalent Bonds
When atoms with significantly different electronegativities bond, the more electronegative atom pulls the shared electrons closer to itself. This creates an unequal distribution of electron density within the molecule. The more electronegative atom develops a partial negative charge (δ-), while the less electronegative atom develops a partial positive charge (δ+). This uneven distribution of charge is the defining characteristic of a polar covalent bond.
Contrasting Polar Covalent Bonds with Other Bond Types
It's important to distinguish polar covalent bonds from other types of chemical bonds:
-
Nonpolar Covalent Bonds: In nonpolar covalent bonds, electrons are shared equally between atoms of similar electronegativity. Examples include bonds between two identical atoms, such as in diatomic molecules like O<sub>2</sub> and N<sub>2</sub>, or bonds between atoms with very small electronegativity differences.
-
Ionic Bonds: Ionic bonds involve the complete transfer of electrons from one atom to another, resulting in the formation of ions (charged particles). This typically occurs between atoms with large electronegativity differences, usually a metal and a nonmetal. The resulting electrostatic attraction between oppositely charged ions forms the ionic bond.
-
Metallic Bonds: Metallic bonds occur in metals and involve the delocalization of valence electrons across a lattice of metal atoms. These delocalized electrons are not associated with any particular atom but contribute to the overall bonding within the metal.
The key distinction lies in the degree of electron sharing. In polar covalent bonds, electrons are shared unequally; in nonpolar covalent bonds, they are shared equally; and in ionic bonds, electrons are transferred completely.
Properties of Molecules with Polar Covalent Bonds
The presence of polar covalent bonds significantly impacts the properties of molecules:
1. Dipole Moments
Polar molecules possess a dipole moment, a measure of the molecule's overall polarity. The dipole moment is a vector quantity, meaning it has both magnitude and direction. It's represented by the symbol µ (mu) and is expressed in Debye units (D). A molecule's dipole moment depends on both the individual bond dipole moments and the molecular geometry. Symmetrical molecules, even with polar bonds, can have a net dipole moment of zero because the individual bond dipoles cancel each other out. Examples include CO<sub>2</sub> (linear) and CCl<sub>4</sub> (tetrahedral). Asymmetrical molecules typically possess a net dipole moment.
2. Solubility
Polar molecules are generally soluble in polar solvents (like water) but insoluble in nonpolar solvents (like oil). This is due to the interaction between the partial charges of the polar molecule and the polar solvent molecules. The partial positive charge of a polar molecule is attracted to the partially negative oxygen atoms in water, while the partially negative charge is attracted to the partially positive hydrogen atoms. This interaction is known as hydrogen bonding when hydrogen is involved.
3. Boiling and Melting Points
Polar molecules tend to have higher boiling and melting points than nonpolar molecules of similar molecular weight. This is because the dipole-dipole interactions between polar molecules are stronger than the weak London dispersion forces that exist between nonpolar molecules. These stronger intermolecular forces require more energy to overcome, resulting in higher boiling and melting points.
4. Reactivity
The polarity of a molecule can influence its reactivity. The partial charges on polar molecules can make them more susceptible to attack by other molecules or ions. This is especially true in chemical reactions involving nucleophiles (electron-rich species) and electrophiles (electron-deficient species).
Examples of Polar Covalent Bonds and Molecules
Numerous examples illustrate polar covalent bonding in various contexts:
-
Water (H<sub>2</sub>O): The oxygen atom in water is significantly more electronegative than the hydrogen atoms. This leads to polar O-H bonds, resulting in a bent molecular geometry and a significant dipole moment. Water's polarity is responsible for many of its unique properties, including its high boiling point, high surface tension, and ability to act as a universal solvent.
-
Hydrogen Fluoride (HF): Fluorine is the most electronegative element, leading to a highly polar H-F bond. The large difference in electronegativity between hydrogen and fluorine results in a very strong dipole moment.
-
Ammonia (NH<sub>3</sub>): The nitrogen atom in ammonia is more electronegative than the hydrogen atoms, creating polar N-H bonds. The pyramidal geometry of ammonia contributes to its overall polarity.
-
Hydrogen Chloride (HCl): The chlorine atom is much more electronegative than the hydrogen atom, creating a highly polar bond. This polarity is reflected in the strong dipole moment and the solubility of HCl in polar solvents.
-
Many Organic Molecules: Numerous organic molecules contain polar covalent bonds, influencing their solubility, reactivity, and other properties. For instance, alcohols (containing -OH groups), aldehydes (containing -CHO groups), and ketones (containing -C=O groups) all have polar bonds that affect their behavior.
Applications of Understanding Polar Covalent Bonds
Understanding polar covalent bonds is essential in various scientific and technological fields:
-
Drug design: The polarity of drug molecules influences their absorption, distribution, metabolism, and excretion (ADME) properties. Designing drugs with appropriate polarity is crucial for achieving the desired therapeutic effect.
-
Materials science: The properties of materials are often determined by the types of bonds present. Understanding polar covalent bonding helps in designing materials with specific properties, such as high dielectric constants or specific solubility characteristics.
-
Environmental chemistry: The polarity of pollutants impacts their environmental fate and transport. Polar pollutants often dissolve readily in water, leading to their accumulation in aquatic systems.
-
Biochemistry: Polar covalent bonds are ubiquitous in biological molecules, including proteins, carbohydrates, and nucleic acids. The polarity of these molecules influences their structure, function, and interactions within cells.
Conclusion
Polar covalent bonding, driven by differences in electronegativity, is a fundamental concept in chemistry with wide-ranging implications. Understanding the principles of polar covalent bonding allows us to predict and interpret the properties of molecules and their behavior in various chemical and biological systems. The unequal sharing of electrons is not simply an academic concept; it’s a key factor influencing a vast array of phenomena, from the solubility of everyday substances to the intricate workings of life itself. The ongoing research into polar covalent bonds continues to broaden our understanding of the chemical world and provides crucial insights for advancements in diverse fields, reinforcing its significance in the broader scientific landscape.
Latest Posts
Latest Posts
-
What Is A Solution To An Equation
Mar 29, 2025
-
This Is Water By David Foster Wallace Pdf
Mar 29, 2025
-
Type I And Type Ii Errors Examples
Mar 29, 2025
-
In Cellular Respiration Most Atp Molecules Are Produced By
Mar 29, 2025
-
What Is The Power Stroke In Muscle Contraction
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Type Of Bonding Involves The Unequal Sharing Of Electrons . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
