All Carbon Carbon Bonds In Benzene Are
Muz Play
Mar 24, 2025 · 6 min read
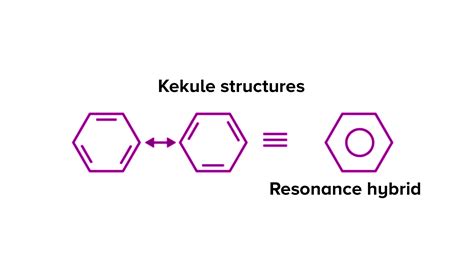
Table of Contents
All Carbon-Carbon Bonds in Benzene Are… Identical! Understanding Benzene's Unique Structure
Benzene, a simple yet fascinating aromatic hydrocarbon, has captivated chemists for centuries. Its unique properties stem from a distinctive feature: all carbon-carbon bonds in benzene are identical. This seemingly straightforward statement belies a complex reality, a blend of structure, bonding, and resonance that underpins its exceptional reactivity and stability. This article delves deep into the nature of these bonds, exploring the concepts of resonance, delocalization, and the implications for benzene's chemical behavior.
The Puzzle of Benzene's Structure: Early Attempts and Kekulé's Proposal
Early attempts to understand benzene's structure were hampered by its unexpected stability. The empirical formula, C₆H₆, suggested a highly unsaturated molecule, prone to addition reactions. Yet, benzene exhibited a surprising resistance to addition and instead favored substitution reactions. This anomaly puzzled scientists until Friedrich August Kekulé proposed his revolutionary model in 1865.
Kekulé suggested a cyclic structure with alternating single and double carbon-carbon bonds, a structure now famously associated with his dream of a snake biting its own tail. This model, while groundbreaking, still couldn't fully explain benzene's unusual properties. If benzene truly possessed alternating single and double bonds, one would expect variations in bond lengths and reactivity. Experimental evidence, however, demonstrated that all six carbon-carbon bonds in benzene are of equal length – an intermediate between a single and a double bond. This discrepancy highlighted a fundamental limitation of Kekulé's model.
The Concept of Resonance: Beyond Kekulé Structures
The solution to this puzzle lies in the concept of resonance. Benzene isn't accurately represented by a single Kekulé structure. Instead, it exists as a hybrid of two equivalent contributing structures, constantly interconverting. These structures, often depicted as rapidly fluctuating between single and double bonds, are not independent entities but rather contribute to a more accurate representation of the molecule’s true structure.
This delocalization of electrons creates a stable ring of electron density above and below the plane of the carbon atoms. This electron cloud is responsible for the unique properties of benzene. It's crucial to understand that benzene is not rapidly switching between the two Kekulé structures; it is a resonance hybrid, a more stable structure than either of the contributing forms.
Visualizing Resonance: The Resonance Hybrid
Think of the resonance hybrid as an average of the two contributing Kekulé structures. The electrons aren't localized in specific double bonds; they are spread out evenly across the entire ring. This results in bond lengths that are equal and intermediate between those of a typical single and double carbon-carbon bond. This delocalization is a key element in explaining benzene's stability and unique reactivity.
The resonance hybrid is often represented by a hexagon with a circle inside, symbolizing the delocalized π electron system. This simplified representation effectively captures the essential feature of benzene’s structure: the complete delocalization of the six π electrons above and below the plane of the ring.
Delocalization: The Key to Benzene's Stability
The stability of benzene is directly linked to the delocalization of its π electrons. In isolated double bonds, the electrons are localized between two carbon atoms. In benzene, however, the six π electrons are shared equally among all six carbon atoms, forming a continuous cloud of electron density.
This delocalization significantly lowers the molecule's overall energy. A localized double bond system would have higher energy. The delocalized system is more stable and thus less reactive than expected based on the presence of six π electrons. This explains benzene's resistance to addition reactions, a property not predicted by the individual Kekulé structures.
Comparing Benzene's Bonds to Single and Double Bonds
It's important to differentiate benzene's carbon-carbon bonds from typical single and double bonds.
-
Single C-C bond: A single bond is formed by the overlap of two sp³ hybridized orbitals, resulting in a sigma (σ) bond. This bond is relatively long and weak.
-
Double C=C bond: A double bond consists of one sigma (σ) bond and one pi (π) bond. The π bond is formed by the side-on overlap of two p orbitals, resulting in a stronger and shorter bond.
-
Benzene C-C bond: Benzene's bonds are intermediate in length and strength between single and double bonds. They are often described as 1.5 bonds, reflecting the partial double bond character due to electron delocalization. The bond order is 1.5, emphasizing the equal sharing of π electrons among all carbon atoms.
Implications for Benzene's Reactivity: Electrophilic Aromatic Substitution
The delocalized π electron system in benzene significantly influences its reactivity. While resistant to addition reactions, benzene readily undergoes electrophilic aromatic substitution reactions. In these reactions, an electrophile (an electron-deficient species) attacks the benzene ring, replacing one of the hydrogen atoms. The delocalized electron system facilitates this substitution, ensuring that the aromatic stability is maintained throughout the reaction mechanism.
The mechanism typically involves the following steps:
-
Electrophilic attack: The electrophile attacks the π electron cloud, forming a carbocation intermediate.
-
Proton abstraction: A proton is abstracted from the carbocation, restoring the aromaticity and forming the substituted benzene derivative.
The stability of the aromatic system is paramount in these reactions. The intermediate carbocation, although destabilized by the positive charge, is stabilized by resonance, preventing the addition reaction which would destroy the aromaticity.
Benzene's Significance in Organic Chemistry and Beyond
Benzene's unique structure and properties are fundamental to organic chemistry. It serves as a building block for a vast array of compounds, including pharmaceuticals, plastics, dyes, and many other essential materials. Understanding its bonding is crucial for comprehending the reactivity and synthesis of a wide range of organic molecules.
Furthermore, benzene’s study has significantly advanced our understanding of chemical bonding and molecular structure. The concept of resonance, initially developed to explain benzene's behavior, has become a fundamental principle in chemistry, used to explain the properties of numerous molecules beyond the realm of aromatics.
Conclusion: The All-Encompassing Uniqueness of Benzene's Bonds
In conclusion, the statement "all carbon-carbon bonds in benzene are identical" reflects a profound truth about this remarkable molecule. The equal bond lengths and bond strengths are a consequence of the delocalized π electron system, a feature that underpins benzene's extraordinary stability and unique reactivity. The concept of resonance, crucial to understanding benzene's structure, is a cornerstone of modern chemistry, highlighting the importance of considering molecular structures as resonance hybrids rather than static representations. The study of benzene continues to be a vital area of research, offering valuable insights into the intricacies of chemical bonding and the synthesis of a wide variety of useful compounds. The simple elegance of benzene’s structure belies its profound impact on chemistry and beyond.
Latest Posts
Latest Posts
-
Biology Word That Starts With J
Mar 25, 2025
-
What Is The Instrument To Measure Humidity
Mar 25, 2025
-
El Preterito De Los Verbos Regulares
Mar 25, 2025
-
Do Transition Metals Have A Charge
Mar 25, 2025
-
How To Calculate The Potential Difference
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about All Carbon Carbon Bonds In Benzene Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
